I����ҵ����CO����ȼ�ϼ״���һ�������·�����Ӧ��CO��g��+2H
2��g��?CH
3OH��g����
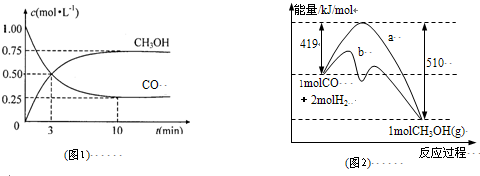
��1��ͼ1�Ƿ�ӦʱCO��CH
3OH��g����Ũ����ʱ��仯������ӷ�Ӧ��ʼ��ƽ�⣬��COŨ�ȱ仯��ʾƽ����Ӧ����v��CO��=
0.075mol?L-1?min-1
0.075mol?L-1?min-1
��
��2��ͼ2��ʾ�÷�Ӧ���й����������ı仯������a��ʾ��ʹ�ô���ʱ��Ӧ�������仯������b��ʾʹ�ô�����������仯���÷�Ӧ��
����
����
��ѡ����ȡ����ȡ�����Ӧ��д����Ӧ���Ȼ�ѧ����ʽ
CO��g��+2H2��g���TCH3OH��g����H=-91KJ/mol
CO��g��+2H2��g���TCH3OH��g����H=-91KJ/mol
��ѡ�����˵Ĵ�����
����
����
����ܡ����ܡ����ı�÷�Ӧ�ķ�Ӧ�ȣ�
��3���÷�Ӧƽ�ⳣ��K�ı���ʽΪ
���¶����ߣ�ƽ�ⳣ��K
��С
��С
������������䡱��С������
��4�����������£����д�ʩ����ʹ
�������
c
c
��
a�������¶ȣ� b������He�� c���ٳ���1molCO��2molH
2 d��ʹ�ô���
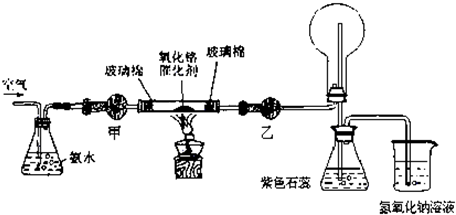
II����13�֣�ij��ѧ����С�����ʵ��̽�����Ļ���������ʣ�װ������ͼ��ʾ��Aװ��δ������������AΪ���巢��װ�ã�A�������Լ������й���������ѡȡ��a��NH
4HCO
3��b��NH
4Cl��c��Ca��OH��
2��d��NaOH��
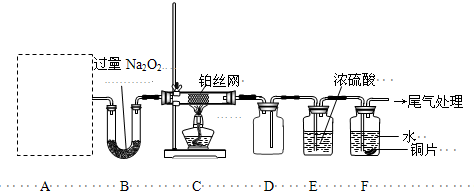
���װ�������Ժ��Ƚ�C����˿�����������ȣ��ٽ�A������������ͨ��Bװ��Ƭ�̺�ȥC���ƾ��ƣ�����ʵ���������£���˿�������ֺ��ȣ�F��ͭƬ���ܽ⣮
��1��ʵ������ȡA������ʱ��ֻ��һ���Լ������Լ���
a
a
���ѡ�Լ�����ĸ������ʱA����Ҫ�IJ���������
�Թܡ��ƾ��ƣ����ܣ�
�Թܡ��ƾ��ƣ����ܣ�
�������ƣ���
��2��A�в��������ʱ�B��Na
2O
2������գ�д������һ��B�з�����Ӧ�Ļ�ѧ����ʽ��
2Na2O2+2H2O=4NaOH+O2����2Na2O2+2CO2=2Na2CO3+O2
2Na2O2+2H2O=4NaOH+O2����2Na2O2+2CO2=2Na2CO3+O2
��
��3������C�з����Ŀ��淴Ӧ������˵����ȷ����
a
a
��
a������һ�ַ�Ӧ���Ũ�ȿ��������һ�ַ�Ӧ���ת����
b����ҵ�Ͻ��и÷�Ӧʱ���ɲ�ȡ��ѹ��������߷�Ӧ��ת����
c���÷�Ӧ��һ�������´ﵽƽ��ʱ����Ӧ���ƽ��Ũ��֮��һ����4��5
��4������ͼ�����л���Cװ���з�Ӧ���������е������仯ʾ��ͼ�����������Ϸֱ�����Ӧ���������Ļ�ѧʽ��
��5����ʵ�������B�й�����������500mL1mol?L
-1 �����У�������ɫ�������ף���Һ�����ԣ���ʵ��ǰB��ԭ��Na
2O
2�����ʵ�����
0.25
0.25
mol�����ڱ�״������
2.8
2.8
L������������ܽ⣩��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�