
��C6H5ONa+H2O+CO2![]() C6H5OH+NaHCO3
C6H5OH+NaHCO3
��C6H5COONa+C6H5OH![]() C6H5COOH+C6H5CONa
C6H5COOH+C6H5CONa
��C6H5COONa+H2O+CO![]() C6H5COOH+NaHCO3
C6H5COOH+NaHCO3
��C5H5COONa+HCl![]() C6H5COOH+NaCl
C6H5COOH+NaCl
A.�٢� B.�ڢ� C.�٢� D.�ڢ�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�������ж�����2011�����һģ���ۻ�ѧ���� ���ͣ�013
|
������֪�����������ʵ�����ǿ���ж�����ȷ���� | |
| [����] | |
A�� |
��֪2C��SiO2 |
B�� |
��֪��ͬ��������̬�⻯����ȶ���HA��HB��˵����ԭ�ԣ�A����B�� |
C�� |
��֪ |
D�� |
��֪��ͬ���ʵ���Ũ��MCl��NCl������Һ��c(M+)��c(N+)��˵�����ԣ�MOH��NOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��ͬ���ʵ���Ũ�ȵ�����������Һ�����������Ҽ����������������̼����ӣ�NaHCO3�����ж����з���ʽ��ȷ���ǣ� ��
��C6H5ONa+H2O+CO2![]() C6H5OH+NaHCO3
C6H5OH+NaHCO3
��C6H5COONa+C6H5OH![]() C6H5COOH+C6H5CONa
C6H5COOH+C6H5CONa
��C6H5COONa+H2O+CO2![]() C6H5COOH+NaHCO3
C6H5COOH+NaHCO3
��C5H5COONa+HCl![]() C6H5COOH+NaCl
C6H5COOH+NaCl
A.�٢� B.�ڢ� C.�٢� D.�ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���ӱ�ʡģ���� ���ͣ���ѡ��
 Si+2CO��˵���ǽ����ԣ�̼>��
Si+2CO��˵���ǽ����ԣ�̼>��  ��˵�����ԣ�̼��>����
��˵�����ԣ�̼��>�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�챱����101��ѧ������ѧ��ͳ������ѧ�Ծ� ���ͣ���ѡ��
������֪�����������ʵ�����ǿ���жϲ���ȷ����
A����֪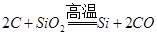 ��˵���ǽ����ԣ�̼>�� ��˵���ǽ����ԣ�̼>�� |
B����֪��ͬ��������̬�⻯����ȶ���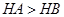 ��˵����ԭ�ԣ� ��˵����ԭ�ԣ� |
C����֪ ��˵�����ԣ�̼��>���� ��˵�����ԣ�̼��>���� |
D����֪��ͬ���ʵ���Ũ�ȵ�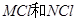 ����Һ�� ����Һ��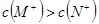 ��˵�����ԣ� ��˵�����ԣ�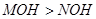 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com