
����þ[Mg(ClO3)2]����������������ݼ��ȣ�ʵ�����Ʊ�����Mg(ClO3)2��6H2O���������£�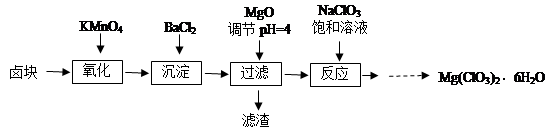
��֪����±����Ҫ�ɷ�ΪMgCl2��6H2O������MgSO4��FeCl2�����ʡ�
�����ֻ�������ܽ��(S )���¶�(T )�仯������ͼ��ʾ��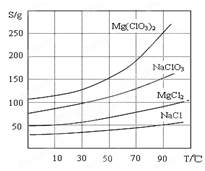
��1����������Ҫ����Ҫ���������� ��
��2������BaCl2��Ŀ���� ����MgO�����������������Ҫ�ɷ�Ϊ ��
��3������NaClO3������Һ������Ӧ�Ļ�ѧ����ʽΪ
��4����Ʒ��Mg(ClO3)2��6H2O�����IJⶨ��
����1��ȷ����3.50 g��Ʒ���100 mL��Һ��
����2��ȡ10.00 mL����ƿ�У�����10.00 mLϡ�����20 .00mL 1.000 mol��L��1��FeSO4��Һ���ȡ�
����3����ȴ�����£���0.100 mol��L��1 K2Cr2O7 ��Һ�ζ�ʣ���Fe2�����յ㣬�˹����з�Ӧ�����ӷ���ʽΪ��Cr2O72����6Fe2����14H����2Cr3����6Fe3����7H2O��
����4��������2��3�ظ����Σ�ƽ������K2Cr2O7 ��Һ15.00 mL��
��д������2�з�����Ӧ�����ӷ���ʽ�� ��
�ڲ�Ʒ��Mg(ClO3)2��6H2O����������Ϊ ��
��1��©�������������ձ�
��2����ȥSO42�� BaSO4��Fe(OH)3
��3��MgCl2��2NaClO3��Mg(ClO3)2��2NaCl����δд�������Ų����֣�
��4����ClO3�� ��6Fe2����6H����6Fe3����Cl����3H2O ��78.3%
���������������1����������Ҫ����Ҫ����������©�������������ձ�����2������BaCl2��Ŀ����ʹ����SO42-ת��Ϊ������ȥ����MgO������Һ��PH��4����ʱFe3+���γ�Fe(OH)3����.��ͬ����BaCl2����������ᱵ����һ����˳�ȥ�����Թ���������������Ҫ�ɷ�ΪBaSO4��Fe(OH)3����3������NaClO3������Һ������Ӧ�Ļ�ѧ����ʽΪMgCl2��2NaClO3��Mg(ClO3)2��2NaCl������Mg(ClO3)2����Һ�еõ�Mg(ClO3)2��6H2O���������ܽ�Ƚϴ����¶ȵ�Ӱ��仯�ϴ���ص��������ᾧ���ٳ��ȹ��ˣ������ȴ�ᾧ�õ�����4������������ԭ��Ӧ���������õ��ĵ����뻹ԭ��ʧȥ�ĵ�������������м��㡣��д������2�з�����Ӧ�����ӷ���ʽ��ClO3�� ��6Fe2����6H����6Fe3����Cl����3H2O������10.00 mL��Һ��1��0.02=3��2��0.015��0.1+ 6��n(ClO3�� ).���n(ClO3�� )="(" 0.011/6)mol.����3.50 g��Ʒ�к��е�Mg(ClO3)2��6H2O����Ϊ{( 0.011/6)mol��2}��10��299g/mol=2.741g.���Բ�Ʒ��Mg(ClO3)2��6H2O����������Ϊ(2.741g��3.50 g)��100��=78.3%
���㣺���鳣��������������ݼ�������þ[Mg(ClO3)2]����ȡ���ɷֲⶨ��֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���ᣨH2C2O4����һ����Ҫ���л�����ԭ�ϡ�Ϊ̽���������ȡ�Ͳ�������ʣ���������ʵ�顣
ʵ���ʵ������������������ˮ��Һ���Ʊ����ᣬװ������ͼ��ʾ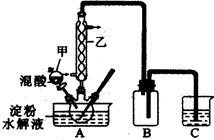
��һ�����ĵ���ˮ��Һ����������ƿ��
�ڿ��Ʒ�Ӧ�¶�55��60�棬�߽�������μ�һ�����Ļ���ᣨ65����HNO3��98����H2SO4��������2�U1.25��
�۷�Ӧ3Сʱ����ȴ�����˺����ؽᾧ�õ����ᾧ��
������������ˮ��Һ�ķ�ӦΪ��
C6H12O6��12HNO3 3H2C2O4��9NO2����3NO����9H2O
3H2C2O4��9NO2����3NO����9H2O
��1����ͼʵ��װ���������ҵ�����Ϊ��________________________��װ��B�������� ��
��2����������Ƿ�ˮ����ȫ�ķ�����______________________________________________��ʵ���̽�����������Ը�����صķ�Ӧ
��3���������Һ����μ��������ữ�ĸ��������Һʱ���ɹ۲쵽��Һ���Ϻ�ɫ��Ϊ������ɫ��д��������Ӧ�����ӷ���ʽ��____________________________________________________��
��4��ѧϰС���ͬѧ���֣����������Һ����μ��������ữ�ĸ��������Һʱ����Һ��ɫ����������졣Ϊ̽����ԭ��ͬѧ���������¶Ա�ʵ�飻
�ɴ�����Ϊ��Һ��ɫ������������ԭ����_________________________________________��
��5�����������ڹ�ҵ������Ҫ���ã���������Ʊ������������������£�
��ȡFeSO4��7H2O ������С�ձ��У�����ˮ������ϡH2SO4��Һ�ữ�������ܽ⡣�����Һ�м���һ������H2C2O4��Һ���������Һ�������У����Ͻ��裬���Ⱪ�У����л�ɫ�����������������á��������Һ���ټ�������ˮ�������ȣ����ˣ����ϴ�ӳ��������ˣ��ñ�ͪϴ�ӹ������β����ɡ�
�����ɵIJ�����������ϴ�ӳ����������Ƿ�ϴ����ȫ�ķ����� ��
���ñ�ͪϴ�ӹ������ε�Ŀ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�о���ѧϰС����ʵ��ʱżȻ���֣������ȥ����Ĥ��þƬ����NaHCO3��Һ��Ӧ������������Ͱ�ɫ�������С��ͬѧͨ������ʵ�飬��֤���ﲢ̽����Ӧԭ����
ʵ��٣���ɰֽ��ȥþ����������Ĥ���������ʢ�������з�̪��Һ�ı���̼��������Һ���ձ��У�Ѹ�ٷ�Ӧ�������������ݺͰ�ɫ�������Һ��dz��ɫ���
����ʵ��
��1������ļ���
ʵ��ڣ���ʵ������ռ����������ȼ���������尲��ȼ�գ�����ʵ���ɫ��������Ϊ ��
��2����С��ͬѧ�Է�Ӧ�в����İ�ɫ�������������²²⣺
�²�1����ɫ���������Ϊ����������
�²�2����ɫ���������ΪMgCO3
�²�3����ɫ���������Ϊ��ʽ̼��þ[yMg(OH)2?xMgCO3]
�����һ��ʵ�����������Ƿ���MgCO3��д��ʵ�����������ͽ��ۣ� ��
��3��ʵ��ۣ�ȡʵ����е���Һ�������м�������CaCl2ϡ��Һ��������ɫ��������Һ��ɫ��dz��˵����Һ�д���CO32�����ӡ�
����ʵ��
��4��Ϊ��һ��ȷ��ʵ��I�İ�ɫ������ijɷ֣��������¶�ʵ�飬װ����ͼ��ʾ��
��ȡ��������İ�ɫ������ 7.36 g����ּ��������ٲ�������Ϊֹ����ʹ�ֽ����������ȫ������װ��A��B�С�ʵ���װ��A����0.72g��װ��B����2.64 g����ɫ������Ļ�ѧʽΪ ��
��5��д��þ�뱥��̼��������Һ��Ӧ�Ļ�ѧ����ʽ ��
��Ӧԭ������
��6��NaHCO3��Һ�д������µ���ƽ�⣺H2O H+ + OH����HCO3��
H+ + OH����HCO3�� H+ +CO32�������ƽ���ƶ��Ƕȷ���ʵ��ٲ�����������Ͱ�ɫ�������ԭ�� ��
H+ +CO32�������ƽ���ƶ��Ƕȷ���ʵ��ٲ�����������Ͱ�ɫ�������ԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�������ױ���ҽҩ��Ⱦ�ϵȹ�ҵ��һ����Ҫ�л��м��壬������Ũ����Ϊ��������Ũ����Ϊ������ͨ���ױ���������Ӧ�Ʊ���
һ���µ��Ʊ��������ױ���ʵ�鷽���ǣ��Է�������Ϊ������������NaHSO4Ϊ����(��ѭ��ʹ��)����CCl4��Һ�У�����������(����ˮ����)��45 �淴Ӧ1 h����Ӧ�������ˣ���Һ�ֱ���5% NaHCO3��Һ��ˮϴ�����ԣ��پ������ᴿ�õ��������ױ���
(1)����ʵ���й��˵�Ŀ����_____________________________________��
(2)��Һ�ڷ�Һ©����ϴ�Ӿ��ú��л��㴦��________��(��ϡ����¡�)����Һʱ��������Һ�����������������ԭ�����Һ©�����������⣬����_________________________________________________________��
(3)���и����˴������༰�����Լױ�������ӦӰ���ʵ������
| ���� |  | ���������и����칹����������(%) | �ܲ���(%) | ||
| �������ױ� | �������ױ� | �������ױ� | |||
| ŨH2SO4 | 1.0 | 35.6 | 60.2 | 4.2 | 98.0 |
| 1.2 | 36.5 | 59.5 | 4.0 | 99.8 | |
| NaHSO4 | 0.15 | 44.6 | 55.1 | 0.3 | 98.9 |
| 0.25 | 46.3 | 52.8 | 0.9 | 99.9 | |
| 0.32 | 47.9 | 51.8 | 0.3 | 99.9 | |
| 0.36 | 45.2 | 54.2 | 0.6 | 99.9 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijͭ��ʯ��ͭԪ�غ����ϵͣ��Һ�������þ���Ƶ����ʡ�ijС����ʵ�������ý�������ȡ���Ʊ�����ͭ��
(1)������Ϊ________���������õ��IJ����������ձ���________��
(2)�������������ҪĿ����________������ͭԪ�ء�
(3)С���Ա����CuSO4��Һ��Na2CO3��Һ��Ϸ�Ӧ���Ʊ�������ľ�ķ�����Cu2(OH)2CO3����Һ�����ʵ�鷢��������ɫ����Һ��ɫ���в��죬�������ϱ��������������������Ʋ�ͬʹ���л��н϶�Cu(OH)2��Cu4(OH)6SO4��
��֪Cu(OH)2��Cu2(OH)2CO3��Cu4(OH)6SO4��������ˮ����������ֽ��¶�����Ϊ80 �桢200 �桢300 �档
���ʵ���������Һ�ɷ֣���ɱ������ݡ�
��ѡ�Լ���2 mol��L��1���ᡢ1 mol��L��1 H2SO4��0.1 mol��L��1 NaOH��Һ��0.1 mol��L��1 BaCl2��Һ������ˮ����������Ʒ��ѡ��
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ��������Һ�����ˣ����ϴ�Ӻ�ȡ�������Թ��У�______________________ | ____________��˵������Һ�л���Cu4(OH)6SO4 |
| ����2����ȡ��������Һ���Թ��У�____________________ | ____________��˵������Һ�л���Cu(OH)2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�̷����壨FeSO4��7H2O��M��278g/mol��������ȱ����ƶѪҩƷ����Ҫ�ɷ֡�ʵ�����������᳧����������Ҫ�ɷ�ΪFe2O3������FeS��SiO2�����Ʊ��̷��Ĺ������£�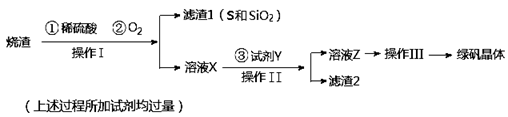
�Իش�
��1������IΪ ����д�������ƣ���
��2���Լ�Y����ҺX��Ӧ�����ӷ���ʽΪ ��
��3������III��˳������Ϊ�� ����ȴ�ᾧ�����ˡ� �����
��4��ijͬѧ������KMnO4��Һ�ⶨ�̷���Ʒ��Fe2+������
a����ȡ11��5g�̷���Ʒ���ܽ⣬���Ƴ�1000mL��Һ��
b����ȡ25��00mL������Һ����ƿ�У�
c���������ữ��0��01000mol/L KMnO4��Һ�ζ����յ㣬����KMnO4��Һ�����ƽ��ֵΪ20��00mL���ٲ���a������Һʱ��Ҫ�IJ�������������������Ͳ���ձ�����ͷ�ι��⣬���� ���ڸ�ͬѧ��Ƶ����еζ���ʽ����������� (�гֲ�����ȥ)(����ĸ���)��
�۵ζ�ʱ������Ӧ�����ӷ���ʽΪ����������������
���жϴ˵ζ�ʵ��ﵽ�յ�ķ����� �����ڵζ��յ��ȡ�ζ��̶ܿ�ʱ������KMnO4��ҺҺ�棬������������ȷ����ʹ�ⶨ��� ���ƫ�ߡ���ƫ�͡�����Ӱ�족����
�ݼ���������Ʒ��FeSO4��7H2O����������Ϊ ��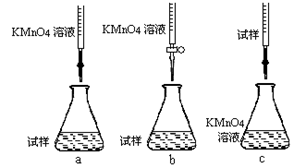
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ͼ��ʵ�����Ʊ�1��2���������鲢����һϵ�����ʵ���װ�ã����ȼ��г��豸���ԣ���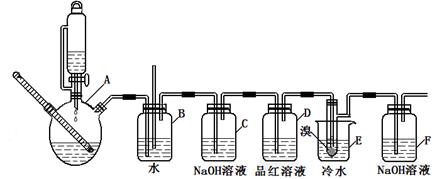
�й������б����£�
| | �Ҵ� | 1,2-�������� | ���� |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶȣ�g/cm3 | 0.79 | 2.2 | 0.71 |
| �е㣯�� | 78.5 | 132 | 34.6 |
| �۵㣯�� | һl30 | 9 | -1l6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�о���ѧϰС������º�ˮ��Һ������CuCl2��2H2O��H2C2O4��2H2OΪԭ�Ϻϳ���ӱ�ṹ�IJ���ͭ���塣Ϊ��һ��̽������ͭ��������ʣ��ֽ�����ͭ������һ�������¼��ȷֽ⣬�������������ͺ�ɫ����������ʵ����о���
��������װ�ü����������ijɷ֡�
��1��Cװ�õ������Ǽ��� ��Dװ�õ������Ǽ��� ��(�û�ѧʽ�ش�)
��2����PdCl2��Һ����COʱ������Ӧ�Ļ�ѧ����ʽΪ______________________________��
��3��D��E��װ��λ���ܷ���Ϊʲô��_____________________________________��
��4������ⶨ�������ĺ�������Aװ���ܺܺõؽ���ʵ������ʵ���У�������____��ʹ�õ�Aװ�ã��ֱ���ʲô���ã�_____________________________��
�Թ������ijɷֽ���̽��
������������裺
����1����ɫ����ΪCu ��
����2����ɫ����ΪCu2O ��
����3�� ��
�����ʵ�鷽��֤����ļ���(��Ҫ�ڴ��������)
��ʵ�����
���ݢ��з�������ʵ���ڴ���ϰ��±��ĸ�ʽд��ʵ�鲽�衢Ԥ����������ۡ�
| ʵ�鲽�� | Ԥ����������� |
| ����1�� | |
| ����2�� | |
| ���� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ���Ҳ���MgCl2��AlCl3�Ļ����Һ�������ˮ��Ӧ�Ʊ�MgAl2O4����Ҫ��������:
(1)�Ʊ�MgAl2O4�����У����±���ʱ������Ӧ�Ļ�ѧ����ʽ ��
(2)��ͼ��ʾ�����˲����е�һ�������� ��
�ж������г����Ƿ�ϴ�����õ��Լ��� �����±���ʱ������ʢ�Ź�������������� ��
(3)��25���£���Ũ�Ⱦ�Ϊ0��01 mol?L-1��MgCl2��AlCl3�����Һ����μ��백ˮ��������__________����(�ѧʽ)�����ɸó��������ӷ���ʽ______________����֪25��ʱKsp[Mg(OH)2]=1.8��10-11��Ksp[Al(OH)3]="3��10" -34����
(4)��ˮAlCl3(183������)����ʪ��������������������ʵ���ҿ�������װ���Ʊ���
װ��B��ʢ�ű���NaCl��Һ,��װ�õ���Ҫ������ ��F���Լ��������� ����һ������װ���ʵ��Լ���Ҳ����F��G�����ã���װ����Լ�Ϊ _��
(5)��Mg��Cu��ɵ�1.96g�����Ͷ�����ϡ�����У���ַ�Ӧ������ȫ�ܽ�ʱ�ռ�����ԭ����NO����0.896L����״��������Ӧ�����Һ�м���2mol/L��NaOH��Һ80mLʱ��������ǡ����ȫ���������γɳ���������Ϊ g��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com