

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �������↑ʼ����ʱ��pH | �������������ȫʱ��pH | |
| Fe3+ | 1.9 | 3.2 |
| Fe2+ | 7.0 | 9.0 |
| Cu2+ | 4.7 | 6.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���������и߿�Ԥ�⣨�ۺ��⣩��ѧ�� ���ͣ�ʵ����
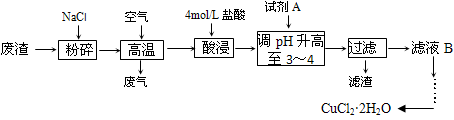
ij���������к��д�����CuS�����������������ҵ���Ը÷�����NaClΪԭ������CuCl2��2H2O���壬�乤����������Ҫ�漰���ա�β�������������������ҺpH�����ˡ������ᾧ�ȡ����չ����з�������Ҫ��ӦΪ�� CuS+2NaCl+2O2 = CuCl2+Na2SO4
��ش��������⣺
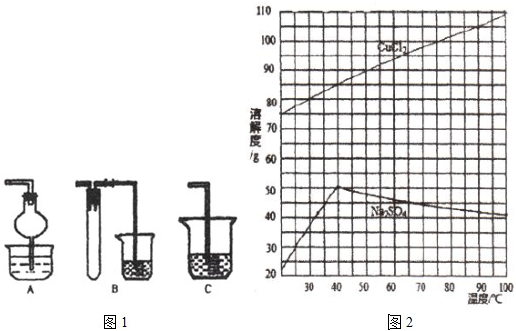
(1)����ʱ���з�������������һ������Ҫ�Ĵ�����Ⱦ�����ʵ�������Լ�Һ���մ���֮������A��B��Cװ���п��е���________(����ĸ)����ѡ�ü�װ�ã����ձ��е��²�Һ�������_______��
(2)������ҺpHʱ����pH��ֽ���ⶨ��Һ��pH������ȷ�IJ���������___________________ ��
(3)��������õ���Һ�е�������ֻ��S042-��Cl-���������Һ�����������ӵ�ʵ�����______ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�츣��ʡܼ����У������ѧ�ڵڶ��ο��Ի�ѧ�Ծ����������� ���ͣ������
��12�֣�
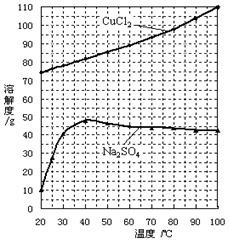
ij���������к��д���CuS���������Ļ������Ԫ�صĻ��ϼ�Ϊ+2��+3�ۣ�����ҵ���Ը÷���Ϊԭ������CuCl2�Ĺ����������£�
���չ����з�������Ҫ��ӦΪ��CuS+2NaCl+2O2����CuCl2+Na2SO4��
�ش��������⣺
��1������ǰ�Ƚ��з����������_____________________________________________��
��2���Լ�AӦѡ�á��� �������ţ���NaClO����Cl2����H2O2��Һ����Ũ����
�����Լ�A��Ŀ����____________________________________________________________��
��3���������˲�������Ҫ�õ��IJ���������_____________________________________________��
��4����CuCl2��Һ�õ�CuCl2����IJ����� ��д���������ƣ����ò���������HCl�����н��У�ԭ����_____________________________________________�������ӷ���ʽ��ʾ����
��5����μ�����ҺB���Ƿ���δ������Fe3+______________________________��
���Լ�A������ҺpH=4ʱ��c(Fe3+)Ϊ_______________������֪Ksp[Fe(OH)3]=2.6��10��39��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡܼ����У������ѧ�ڵڶ��ο��Ի�ѧ�Ծ��������棩 ���ͣ������
��12�֣�
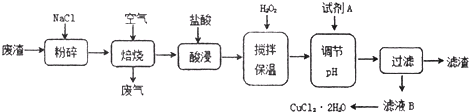
ij���������к��д���CuS���������Ļ������Ԫ�صĻ��ϼ�Ϊ+2��+3�ۣ�����ҵ���Ը÷���Ϊԭ������CuCl2�Ĺ����������£�

���չ����з�������Ҫ��ӦΪ��CuS+2NaCl+2O2����CuCl2+Na2SO4��
�ش��������⣺
��1������ǰ�Ƚ��з����������_____________________________________________��
��2���Լ�AӦѡ�á��� �������ţ���NaClO����Cl2����H2O2��Һ����Ũ����
�����Լ�A��Ŀ����____________________________________________________________��
��3���������˲�������Ҫ�õ��IJ���������_____________________________________________��
��4����CuCl2��Һ�õ�CuCl2����IJ����� ��д���������ƣ����ò���������HCl�����н��У�ԭ����_____________________________________________�������ӷ���ʽ��ʾ����
��5����μ�����ҺB���Ƿ���δ������Fe3+______________________________��
���Լ�A������ҺpH=4ʱ��c(Fe3+)Ϊ_______________������֪Ksp[Fe(OH)3]=2.6��10��39��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com