

(1)������ָ���Ƿ��б仯_______________
(2)��C����Һ�������Һ����______________���йط���ʽΪ_______________________��
(3)�������������________________��
(4)��װ��Ϊ_________�ء�
(5)�ѵ缫��Ϊͭ�缫ʱ�кα仯��
____________________________________________________________________��
(6)����FeCl3��Һ������H2SO4�ữ��KMnO4��Һ��������ָ���кα仯��__________��
д��������Ӧ�Ļ�ѧ����ʽ�����������ת�Ƶķ��������
____________________________________________________________________��
������(1)������ָ��ƫ�ƣ���ΪFeCl3��KI�䷢����������ԭ��Ӧ��ת�Ƶĵ���Ҫͨ�������ơ�
(2)C����Һ�����۱���������ΪFe3+��I-������I2��2FeCl3+2KI![]() 2FeCl2+2KCl+I2��
2FeCl2+2KCl+I2��
(3)��Ϊ![]() �����Ե�����C����B��
�����Ե�����C����B��
(4)�γ��Է���أ���ԭ����ԭ�����ͬ��
(5)�����缫����ͭ�缫ʱ������FeCl3��Һ��ֱ�ӷ���������ԭ��Ӧ��Cu+2Fe3+![]() 2Fe2++Cu2+��ֱ���ܽ⣬���û�е������������ƣ�ָ�벻��ƫ�ơ�
2Fe2++Cu2+��ֱ���ܽ⣬���û�е������������ƣ�ָ�벻��ƫ�ơ�
(6)��Ϊ�����Ի����£�KMnO4��FeCl3��������ǿ��B������Һ�����Ϻ�ɫ����ɫ��C������Һ����ɫ��������ָ��ƫ�Ƴ̶Ⱥܴ�ӦΪ
2KMnO4+10KI+8H2SO4![]() 6K2SO4+2MnSO4+8H2O+5I2
6K2SO4+2MnSO4+8H2O+5I2
��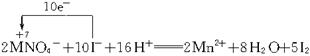
�𰸣�(1)������ָ��ƫ��
(2)C����Һ�����۱���
2FeCl3+2KI![]() 2FeCl2+2KCl+I2
2FeCl2+2KCl+I2
(3)������C����B
(4)ԭ���
(5)����FeCl3��Һ��ֱ�ӷ���������ԭ��Ӧ��Cu+2Fe3+![]() 2Fe2++Cu2+��ֱ���ܽ⣬���û�е������������ƣ�ָ�벻��ƫ��
2Fe2++Cu2+��ֱ���ܽ⣬���û�е������������ƣ�ָ�벻��ƫ��
(6)������ָ��ƫ�Ƴ̶Ⱥܴ�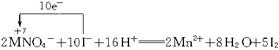



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
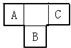 A��B��C��D��EΪ������Ԫ�أ�A��B��C�����ڱ���������λ����ͼ��ʾ��A��C��Ԫ�ص�ԭ�Ӻ��������֮�͵���Bԭ�ӵ���������Bԭ�Ӻ�������������������ȣ�D��Aͬ���ڣ����������������۴�����Ϊ�㣮A��E���γ�AE3�ͷ��ӣ�������ֻ���ڼ��Լ���
A��B��C��D��EΪ������Ԫ�أ�A��B��C�����ڱ���������λ����ͼ��ʾ��A��C��Ԫ�ص�ԭ�Ӻ��������֮�͵���Bԭ�ӵ���������Bԭ�Ӻ�������������������ȣ�D��Aͬ���ڣ����������������۴�����Ϊ�㣮A��E���γ�AE3�ͷ��ӣ�������ֻ���ڼ��Լ��� 



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
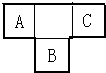 A��B��CΪ������Ԫ�أ������ڱ���������λ����ͼ��ʾ��A��C��Ԫ�ص�ԭ�Ӻ��������֮�͵���Bԭ�ӵ���������Bԭ�Ӻ�������������������ȣ�
A��B��CΪ������Ԫ�أ������ڱ���������λ����ͼ��ʾ��A��C��Ԫ�ص�ԭ�Ӻ��������֮�͵���Bԭ�ӵ���������Bԭ�Ӻ�������������������ȣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣�A��B��CΪ������Ԫ�أ������ڱ���������λ����ͼ��ʾ��A��C��Ԫ�ص�ԭ�Ӻ��������֮�͵���Bԭ�ӵ���������Bԭ�Ӻ��������������������
��1��д��A��B��C����Ԫ�ص����� �� �� ��
��2��BԪ��λ��Ԫ�����ڱ��е� ���ڣ��� �塣
��3��C��ԭ�ӽṹʾ��ͼΪ ���õ���ʽ��ʾC�ĵ�����H2��Ӧ�Ĺ��̣� ��
��4����Ԫ��A��C����Ԫ���γɵĻ������к��еĻ�ѧ���������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ֣���и�����ѧ�ڵ�һ���¿���ѧ�Ծ� ���ͣ������
��10�֣�A��B��CΪ������Ԫ�أ������ڱ���������λ����ͼ��ʾ��A��C��Ԫ�ص�ԭ�Ӻ��������֮�͵���Bԭ�ӵ���������Bԭ�Ӻ��������������������

��1��д��A��B��C����Ԫ�ص����� �� �� ��
��2��BԪ��λ��Ԫ�����ڱ��е� ���ڣ��� �塣
��3��C��ԭ�ӽṹʾ��ͼΪ ���õ���ʽ��ʾC�ĵ�����H2��Ӧ�Ĺ��̣� ��
��4����Ԫ��A��C����Ԫ���γɵĻ������к��еĻ�ѧ���������� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com