

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
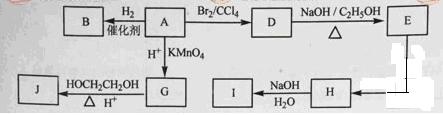
��ͼ��A-J��Ϊ�л����������ͼ�е���Ϣ���ش��������⣺
��1����״������A����Է�������Ϊ82�����к�̼87.80%������12.2%��B��һ�ȴ������һ�֣�B�Ľṹ��ʽΪ ��3�֣�
��2��M��B��һ��ͬ���칹�壬M��ʹ������Ȼ�̼��Һ��ɫ�����������е�̼ԭ�ӹ�ƽ�棬��M�Ľṹ��ʽΪ ��3�֣���
��3����A����D�ķ�Ӧ������ ��3�֣�����D����E�ķ�Ӧ������ ��3�֣���
��4��G�ķ���ʽΪC6H10O4,0.146gG����20ml0.100mol/L NaOH��Һ��ȫ�кͣ�J��һ�ָ߷��ӻ��������Gת��ΪJ�Ļ�ѧ����ʽΪ ��4�֣���
��5��H�к��й������� ��3�֣��� I�к��еĹ������� ��3�֣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ɹż���һ�и߶��ڶ�ѧ����ĩ���Ի�ѧ���� ���ͣ������
|
 ��ͼ��A-J��Ϊ�л����������ͼ�е���Ϣ���ش��������⣺
��ͼ��A-J��Ϊ�л����������ͼ�е���Ϣ���ش��������⣺ A����D�ķ�Ӧ������ ��3�֣�����D����E�ķ�Ӧ������ ��3�֣���
A����D�ķ�Ӧ������ ��3�֣�����D����E�ķ�Ӧ������ ��3�֣����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ɹŸ߶��ڶ�ѧ����ĩ���Ի�ѧ���� ���ͣ������

��ͼ��A-J��Ϊ�л����������ͼ�е���Ϣ���ش��������⣺
��1����״������A����Է�������Ϊ82�����к�̼87.80%������12.2%��B��һ�ȴ������һ�֣�B�Ľṹ��ʽΪ ��3�֣�
��2��M��B��һ��ͬ���칹�壬M��ʹ������Ȼ�̼��Һ��ɫ�����������е�̼ԭ�ӹ�ƽ�棬��M�Ľṹ��ʽΪ ��3�֣���
��3����A����D�ķ�Ӧ������ ��3�֣�����D����E�ķ�Ӧ������ ��3�֣���
��4��G�ķ���ʽΪC6H10O4, 0.146gG����20ml0.100mol/L NaOH��Һ��ȫ�кͣ�J��һ�ָ߷��ӻ��������Gת��ΪJ�Ļ�ѧ����ʽΪ ��4�֣���
��5��H�к��й������� ��3�֣��� I�к��еĹ������� ��3�֣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ��ƶ���


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���߿����� ���ͣ��ƶ���


�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com