
��֪������±�����ڴ��������¿��������������±���⡣��������ת����ϵ�����������������������ȥ�����ش��������⣺
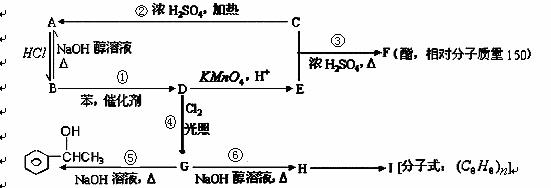
��1��D�Ľṹ��ʽΪ ��E��H�����������ŵ����Ʒֱ��� �� ��
��2���٢ڢۢܢݢ�Ӧ��������ȥ��Ӧ���� ��
��3��д���ķ�Ӧ����ʽ ��
д��H��I�ķ�Ӧ����ʽ ��
��4��������֤G�к�����Ԫ�صķ����� ��
��5��д��һ�ַ�������Ҫ���F��ͬ���칹��Ľṹ��ʽ;
��F��ͬ���칹�����������࣬�ܷ���������Ӧ���ұ����ϵ�һ��ȡ����ֻ�����֣� ��
��F��ͬ���칹�������ڴ��࣬�ܷ���������Ӧ���ұ����ϵ�һ��ȡ����ֻ�����֣� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������м�����Ũ�������ֿ��ù����������Ƹ������
A��NH3 B��H2
C��Cl2 D��SO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ������������أ������й�˵����ȷ����
A��ά����C���л�ԭ�ԣ�������������������
B�����ࡢ�����ʡ���֬������Ȼ�߷��ӻ�����
C��ú��������Һ�����������仯���̣��ɱ�Ϊ�����Դ
D������������ľ�����ά�����ڹ���ͨ�ŵĹ��ά�����������ǽ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ��Ϊ�˲ⶨ��ͭ����Ҫ�ɷ���Cu2S����Cu2S�������������������ͼװ�á�ʵ��ʱ�����²��������
A. ����ȫ��������ʹ���Ϊ��ͼװ�ã������װ�õ������ԡ�
B. ��ȡ��ϸ�Ļ�ͭ����Ʒ1.000g��
C. �������õ���ƷС�ĵط���Ӳ�ʲ������С�
D. ��ÿ����1L�����ʹ��������
E.��Ӳ�ʲ������еĻ�ͭ����Ʒ���ȵ�һ���¶�,������ӦΪ:Cu2S+O2=SO2 +2Cu��
F. ��ȡ25.00ml��SO2��ˮ��Һ��250ml��ƿ�У���0.0100mol/L KMnO4����Һ�ζ����յ㡣���������������ظ��ζ�2��3�Ρ�

�Իش��������⣺
��1��װ�âٵ�������_________________��װ�âڵ�������____________________��
��2���ٶ���ͭ���е���ȫ��ת��ΪSO2������ȫ����ˮ���գ������F����������Ӧ�Ļ�ѧ����ʽΪ ��������______________________________ _������ʱ���жϵζ��Ѿ��ﵽ�յ㡣
��3��������F�ĵζ�������±���ʾ�����ͭ����Ʒ��Cu2S������������_________��
| �ζ� ���� | ������Һ�� ���/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.04 | 21.03 |
| 2 | 25.00 | 1.98 | 21.99 |
| 3 | 25.00 | 3.20 | 21.24 |
��4���������������һ�����Ե�ȱ��Ӱ���˲ⶨ����������ڲ���ʧ������Ϊ��
��дһ�ּȿɣ���
��5����֪�ڳ�����FeS �� Ksp�� 6 . 25 �� 10 ��18, H2S ������Һ�� c (H������ c (S2����֮��������¹�ϵ�� c2 (H��) ����S2��) = 1 . 0��10��22 ���ڸ��¶��£������� FeS Ͷ�����ⱥ����Һ�У���ʹ��Һ�У�Fe2+��Ϊ lmol/L��Ӧ������Һ��c��Hʮ��Ϊ__________________������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и������ʣ�ǰ���ǻ��������Ǵ� ������ǣ� ��
������ǣ� ��
A���������Ȼ��� B������Ũ����
C�������백ˮ D���ɱ���Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ��д��ȷ����( )
A. ��Һ�Լ���:
��Һ�Լ���:


B.̼��������Һ�м����������ռ���Һ: ===
===
C.��������ˮ��Ӧ: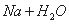 ===
=== ��
��
D. ��FeCl3��Һ��ʴӡˢ��·�壺 Fe3+��Cu==Fe2+��Cu2+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D��E��F��G���Ƕ���������Ԫ�أ����ǵĺ˵������������Ԫ��A��ԭ�Ӻ��ڽ���һ�����ӣ�A��Dͬ���壬B��CΪͬ����Ԫ�أ�����A���γ���ͬ�������Ļ����C��Fͬ���壬F��������ΪC��2����Ԫ��E��������������K���������1��B��C��F��������֮�͵���E��G��������֮�͡���ش��������⣺
(1)д����������������Ԫ����ɵľ���Ư�����õ����ʵĻ�ѧʽ________(����д������)��
(2)A�ֱ���B��C��G���γ���Ӧ��������ֻ���������ֻ�����ķе��ɸߵ��͵�˳��Ϊ__________(�û�ѧʽ��ʾ)��
(3)A��C��G���γ�һ��3ԭ�ӷ��ӣ���д�����ĵ���ʽ ��C��D���γ�һ����Է�������Ϊ78�Ļ�������ĵ���ʽΪ �����������Ļ�ѧ���У� ��
(4) D��E����Ԫ�ص�ԭ�Ӷ����γ���Ӧ�ļ����ӣ��������Ӱ뾶��С��ϵΪ________(�����ӷ��ű�ʾ)����D��E����Ԫ�صĵ���ͬʱͶ��ˮ�У���ַ�Ӧ�����Һ��ֻ��һ�����ʣ�����������ʣ�࣬����Ͷ�뵽ˮ�е�D�ĵ��ʺ�E�ĵ��ʵ�����֮��Ϊ________��������Ӧ�Ļ�ѧ����ʽΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1����һƿ���ʱ��ϳ�����������������ϲ������Ա仯����ijѧ���������в��������������ʣ��������1���������������Ƿ���ʵ�ʵ�鷽���� ������б��ʣ�����������������Һʱ��Ӧ��γ�ȥ���ʵ����ʣ� ����2��FeCl3��Һ�ػ�ɫ���Դ���Һ���ֱ�������ʵ�飬�������
| ��� | ʵ������ | ʵ����Ҫ���� | ���ӷ���ʽ |
| �� | ����������� | ||
| �� | ��������Na2O2��ĩ | ||
| �� | ��������AgNO3��Һ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com