
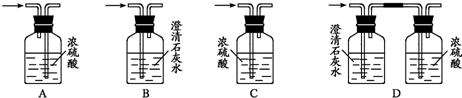
Ϊ��֤���Ҵ������к�����ԭ�ӣ�ijС���������ͼװ�ý���ʵ���о���

ͼ���ձ�A����ˮԡ������ƿB��ʢ���Լ�X����Һ©��C���ƿD�о�ʢ��Ũ���ᣬE��һ��ֱ���ܣ������F���������Ƶļ�ʯ�ң���ƿG��װ����ˮ�Ҵ���һЩ���ư�ɫ��ˮ�η�ĩY����C����������Ũ������������B�����Լ�X���ã�֮����Dƿ�ĵ��ܿڲ����������ݣ�ˮԡ����Gƿ�������ֹ۲쵽Gƿ���Լ�Y��ɫ��F���ų������徭�������ܵ�ȼ����ش��������⣺
��1��Bƿ����װ���Լ�X�����µ�_________����ѡ����ĸ����
A������ʳ��ˮ B��MnO2��NaCl����� C��Ũ����
��2��C��Ũ�������Ҫ������____________________________��B���ݳ�������ɷ���
__________________��
��3��D����װŨ�����������__________________��
��4��Gƿ�з����л���Ӧ�Ļ�ѧ����ʽ��___________________________��
��5��F���������Ƶļ�ʯ�ҵ�Ŀ����__________________________��E���ܳ����е����Ļ��������⣬�ڱ�ʵ���л����е�������___________��
��6����ˮ�η�ĩY�Ļ�ѧʽ��_______________����ʵ����֤���Ҵ������к�����ԭ�ӵ�������_______________________________��
 �����ÿ�ʱѵ��ϵ�д�
�����ÿ�ʱѵ��ϵ�д� ��Ԫȫ��������ϵ�д�
��Ԫȫ��������ϵ�д� �»ƸԱ����ܾ�ϵ�д�
�»ƸԱ����ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| �� |
| ||
| �� |
| ����ø |
| �ƻ�ø |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������ѧ���¿γ̡���ѧ2����(³�ư�) ���ͣ�058
ijУ��ѧ������ȤС���ѧ��Ϊ����֤�Ҵ��ķ��ӽṹ���������ʵ������Ҵ������Ԫ�صIJⶨ������ʽ�IJⶨ�����ӽṹ�IJⶨ��

(1)���Ǿ�����ȼ���Ҵ�����������ȷ���Ҵ��к���C��H����Ԫ�أ���Ҫ˵�����ǵľ��������
��֤��������Ԫ�صIJ�����___________��
��֤������̼Ԫ�صIJ�����___________��
(2)Ҫ��ȼ�շ�������֤ʵ�Ҵ��л�������Ԫ��ʱ����ȡ��һЩʵ�����ݣ���Щ����Ӧ����________��
(3)Ϊȷ���Ҵ��ķ���ʽ����(2)�������⣬���費��Ҫ�ⶨ�Ҵ�����Է���������_______________________��
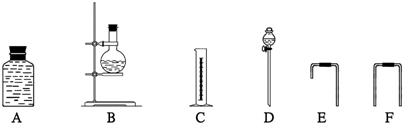
(4)Ϊ�ⶨ�Ҵ����ӽṹ������������ˮ�Ҵ��ͽ����Ʒ�Ӧ�ռ����������ķ�����ѡ��������ͼ��ʾ������(�е���������˫����Ƥ��)��
��װ�õ�����˳����________��________��________��________��________��________��
����ʵ��֤���Ҵ����ӵĽṹ��ʽ��CH3CH2OH������CH3OCH3��������_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣�ijУ��ѧ����С���ѧ��Ϊ����֤�Ҵ��ķ��ӽṹ���������ʵ������Ҵ��ľ��ơ��Ҵ����Ԫ�صIJⶨ������ʽ�IJⶨ�����ӽṹ�IJⶨ��
��1���������Ҵ��к�����ˮ������ֱ������ķ���������Ч��ȥˮ��ͨ�������м��� ��Ȼ������֤���Ҵ����ٺ�ˮ������һ���Լ����飬�����Լ��� ��
��2����ȼ���Ҵ���������ķ���ȷ������C��H��O����Ԫ�ء�
a��֤������HԪ�صIJ����� ��
b��֤������OԪ��ʱ����ȡ�õ�ʵ�������ǣ�
CO2��������H2O�������� ��
��3��Ϊȷ���Ҵ��ķ���ʽ����ͨ��(2)��ȡ�Ҵ���ʵ��ʽ֮���Ƿ�����ٲⶨ�Ҵ�����Է�����������ȷ�������ʽ (���ǡ���)�������� ��
��4���ⶨ�Ҵ��ķ��ӽṹ
a�������ú�������Dzⶨ����ͨ�����ú���������� ���������շ壬����ȷ���Ҵ��Ľṹ��CH3CH2OH������CH3OCH3��(�C��H������C��C������C��O������O��H��)
b�������ú˴Ź����Dzⶨ���������Ҵ��ĺ˴Ź���������Ӧ�� �����շ塣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�켪��ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
ijУ��ѧ������ȤС���ѧ��Ϊ����֤�Ҵ��ķ��ӽṹ���������ʵ������Ҵ����Ԫ�صIJⶨ������ʽ�IJⶨ�����ӽṹ�IJⶨ��
��1�����Ǿ�����ȼ���Ҵ�����������ȷ���Ҵ��к���C��H����Ԫ�ء���Ҫ˵�����ǵľ����������֤��������Ԫ�صIJ�����________________________________________________________ _
________________________________________________________________________________________��
��֤������̼Ԫ�صIJ�����________________________________________________
_______________________________________________________________________
��2��Ҫ��ȼ�շ�������֤ʵ�Ҵ��л�������Ԫ��ʱ����ȡ��һЩʵ�����ݣ���Щ����Ӧ����________________________��
��3��Ϊȷ���Ҵ��ķ���ʽ������2���������⣬���費��Ҫ�ⶨ�Ҵ�����Է�������?
_______________________________________________________________________
��4��Ϊ�ⶨ�Ҵ����ӽṹ������������ˮ�Ҵ��ͽ����Ʒ�Ӧ�ռ����������ķ�����ѡ��������ͼ��ʾ������(�е���������˫����Ƥ��)��

��װ�õ�����˳����______��______��______��______��_______��_______��
����֪��ˮ�ƾ����ܶ�Ϊ0��789 g��cmһ3����ȡ2��0mL�ƾ�����Ӧ��ȫ��(�ƹ���)���ռ�390 mL���塣���Ҵ��������ܱ���ȡ��������ԭ����Ϊ_______���ɴ˿�ȷ���Ҵ��ĽṹΪ______________________������______________��
��5��ʵ�����ⶨ�Ľ��ƫ�ߣ����������ԭ����(��д���)��______________
A����ʵ���������½���
B����ˮ�ƾ��л������״�
C����ˮ�ƾ����Ʒ�Ӧ������ȫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������ʡ�߶��������¿���ѧ�Ծ� ���ͣ�ʵ����
��10�֣�ijУ��ѧ����С���ѧ��Ϊ����֤�Ҵ��ķ��ӽṹ���������ʵ������Ҵ��ľ��ơ��Ҵ����Ԫ�صIJⶨ������ʽ�IJⶨ�����ӽṹ�IJⶨ��
��1���������Ҵ��к�����ˮ������ֱ������ķ���������Ч��ȥˮ��ͨ�������м��� ��Ȼ������֤���Ҵ����ٺ�ˮ������һ���Լ����飬�����Լ��� ��
��2����ȼ���Ҵ���������ķ���ȷ������C��H��O����Ԫ�ء�
a��֤������HԪ�صIJ����� ��
b��֤������OԪ��ʱ����ȡ�õ�ʵ�������ǣ�
CO2��������H2O�������� ��
��3��Ϊȷ���Ҵ��ķ���ʽ����ͨ��(2)��ȡ�Ҵ���ʵ��ʽ֮���Ƿ�����ٲⶨ�Ҵ�����Է�����������ȷ�������ʽ (���ǡ���)�������� ��
��4���ⶨ�Ҵ��ķ��ӽṹ
a�������ú�������Dzⶨ����ͨ�����ú���������� ���������շ壬����ȷ���Ҵ��Ľṹ��CH3CH2OH������CH3OCH3��(�C��H������C��C������C��O������O��H��)
b�������ú˴Ź����Dzⶨ���������Ҵ��ĺ˴Ź���������Ӧ�� �����շ塣
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com