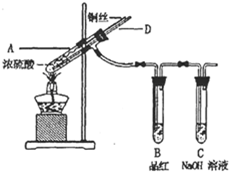
��2012?��ɳģ�⣩ijʵ��С��ͬѧΪ��̽��ͭ��Ũ����ķ�Ӧ������������ϵ��ʵ�飮
��ʵ��1��ͭ��Ũ���ᷴӦ��ʵ��װ����ͼ��ʾ��
ʵ�鲽�裺
�������Ӻ�װ�ã����������ԣ������Լ���
�ڼ���A�Թ�ֱ��B��Ʒ����ɫ��Ϩ��ƾ��ƣ�
�۽�Cu˿�����뿪Һ�森
��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ
Cu+2H
2SO
4��Ũ��
CuSO
4+SO
2��+2H
2O
Cu+2H
2SO
4��Ũ��
CuSO
4+SO
2��+2H
2O
��װ��C�з�����Ӧ�����ӷ���ʽΪ
SO2+2OH-=SO32-+H2O
SO2+2OH-=SO32-+H2O
��
��2��Ϩ��ƾ��ƺ���Ϊ�е���D�Ĵ��ڣ�B�е�Һ�岻�ᵹ������ԭ����
�Թ�A������ѹǿ��С��������D���ܽ����Թ�A��
�Թ�A������ѹǿ��С��������D���ܽ����Թ�A��
��
��3�����װ��ǰ������������Ϳ�ʹװ���в���������ȫ�����գ�Ӧ����ȡ�IJ�����
��D�ܿ���A��ͨ�������������
��D�ܿ���A��ͨ�������������
��
��ʵ��2��ʵ���з����Թ��ڳ��˲�����ɫ�����⣬��ͭ˿���滹������ɫ����ף����п��ܺ�������ͭ����ͭ������ͭ���Լ����ڱε�������ͭ��
�������ϣ�
��������ͭ�����Ի����»ᷢ������������ԭ��Ӧ����Cu
2+��ͭ���ʣ��������������գ�����ת��Ϊ����ͭ��
����ͭ������ͭ�����¶�������ϡ���ᣬ�������������գ���ͭ������ͭ��ת��Ϊ����ͭ�Ͷ�������Ϊ���о��ijɷ֣���С��ͬѧ���ռ����㹻���Ĺ��������ͼ��ʵ�飺
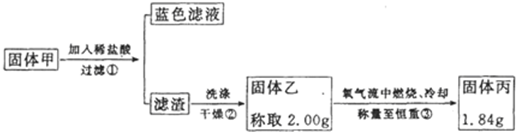
��4�����м��������Ƿ�ϴ�Ӹɾ���ʵ�鷽����
ȡ���һ��ϴ�Ӻ�����Һ�壬�μ���������Һ�����ް�ɫ������������˵������ϴ�Ӹɾ������а�ɫ�������ɣ���˵��δϴ�ɾ�
ȡ���һ��ϴ�Ӻ�����Һ�壬�μ���������Һ�����ް�ɫ������������˵������ϴ�Ӹɾ������а�ɫ�������ɣ���˵��δϴ�ɾ�
��
��5�����������չ�����һ�������ķ�Ӧ�Ļ�ѧ����ʽΪ
��
��6�����ж��ڹ���ijɷֵ��ж��У�����ȷ���ǣ�����ĸѡ�
BCD
BCD
��
A���������CuS��Cu
2S����ͬʱ����
B���������CuO��Cu
2O������һ��
C�����������û��Cu
2O����һ����Cu
2S
D���������������Cu
2O��Ҳ������Cu
2S��

 ��У����ϵ�д�
��У����ϵ�д�

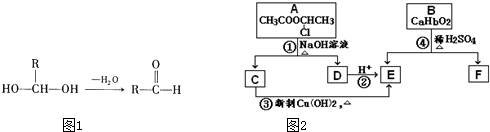
 A��B��C��D��E��FΪ��ѧ��ѧ�еij������ʣ�������A��1��2�ֶ�����Ԫ����ɣ���һ��������������ת����ϵ��������������⣺
A��B��C��D��E��FΪ��ѧ��ѧ�еij������ʣ�������A��1��2�ֶ�����Ԫ����ɣ���һ��������������ת����ϵ��������������⣺ ��2012?��ɳģ�⣩ijʵ��С��ͬѧΪ��̽��ͭ��Ũ����ķ�Ӧ������������ϵ��ʵ�飮
��2012?��ɳģ�⣩ijʵ��С��ͬѧΪ��̽��ͭ��Ũ����ķ�Ӧ������������ϵ��ʵ�飮


 ��
�� ��
�� ��
��
 ��
�� ��
�� ��
��