









 ��A�ڴ������ȵ������·�����ȥ��Ӧ���ɺ���˫��������B��
��A�ڴ������ȵ������·�����ȥ��Ӧ���ɺ���˫��������B�� ��B���Ժ��嵥�ʷ����ӳɷ�Ӧ��C��
��B���Ժ��嵥�ʷ����ӳɷ�Ӧ��C�� �����������Ƶ�ˮ��Һ�У�±��ԭ��ˮ�����ɴ������ʣ��Ȼ��ͼ���кͷ�Ӧ�õ����Σ����õ�D��
�����������Ƶ�ˮ��Һ�У�±��ԭ��ˮ�����ɴ������ʣ��Ȼ��ͼ���кͷ�Ӧ�õ����Σ����õ�D�� �����������Ի������ữ�Ķ�������������E��
�����������Ի������ữ�Ķ�������������E�� ��E�Ļ�ѧʽΪC4H6O6��
��E�Ļ�ѧʽΪC4H6O6�� ��A�ڴ������ȵ������·�����ȥ��Ӧ���ɺ���˫��������B��
��A�ڴ������ȵ������·�����ȥ��Ӧ���ɺ���˫��������B�� ��B���Ժ��嵥�ʷ����ӳɷ�Ӧ��C��
��B���Ժ��嵥�ʷ����ӳɷ�Ӧ��C�� �����������Ƶ�ˮ��Һ�У�±��ԭ��ˮ�����ɴ������ʣ��Ȼ��ͼ���кͷ�Ӧ�õ����Σ����õ�D��
�����������Ƶ�ˮ��Һ�У�±��ԭ��ˮ�����ɴ������ʣ��Ȼ��ͼ���кͷ�Ӧ�õ����Σ����õ�D�� �����������Ի������ữ�Ķ�������������E��
�����������Ի������ữ�Ķ�������������E�� ��E�Ļ�ѧʽΪC4H6O6��
��E�Ļ�ѧʽΪC4H6O6�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��
�� ��
�� ��CH3CH2OH ���Ӽ���ˮ��7������ֱ�Ϊ��
��CH3CH2OH ���Ӽ���ˮ��7������ֱ�Ϊ��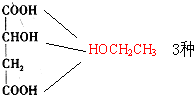
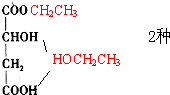

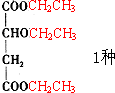 ����7�֣��ʴ�Ϊ��7��
����7�֣��ʴ�Ϊ��7�� ���ʴ�Ϊ��
���ʴ�Ϊ��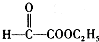 ��
�� ����ˮ����Ի������ʹ�����G��ˮ��Ӧ����A��M�Ļ�ѧ����ʽΪ��
����ˮ����Ի������ʹ�����G��ˮ��Ӧ����A��M�Ļ�ѧ����ʽΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��

 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
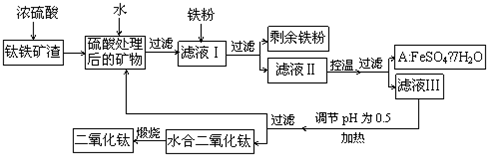
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ �� �� �� | ʵ �� �� �� | ʵ �� �� �� |
| ��֤������ | ||
| ��֤���ȶ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com