
| A�� | ��ȡ12.5g������CuSO4•5H2O�������500mL��Һ | |
| B�� | ��ȡ12.5g������CuSO4•5H2O��������500mLˮ�����Һ | |
| C�� | ��ȡ7.68g��ˮ����ͭ��ĩ������480mLˮ�����Һ | |
| D�� | ��ȡ8.0g��ˮ����ͭ��ĩ������500mLˮ�����Һ |
���� ʵ����û��480mL������ƿ��Ӧѡ�����480mL�ҹ�����������ƿ����Ӧѡ��500mL����ƿ��������Һ�����Ϊ500mL������n=cV��������ͭ�����ʵ�������Ҫ����ͭ���ʵ�����������ͭ��������ʵ������ٸ���m=cVM������������ͭ������������ͭ������������ݴ��жϣ�ע��500mL����Һ������������ܼ������Ϊ500mL��
��� �⣺����ʵ����û��480mL������ƿ��ֻ��ѡ��500mL����ƿ������500mL 0.1mol/L������ͭ��Һ����Ҫ����ͭ�����ʵ���Ϊ��0.1mol/L��0.5L=0.05mol������ͭ������Ϊ��160g/mol��0.05mol=8.0g����Ҫ��������Ϊ��250g/mol��0.05mol=12.5g��
A����ȡ12.5g���������500mL��Һ���õ�����Һ��Ũ��Ϊ0.1mol/L����A��ȷ��
B����ȡ12.5g�������500mL��Һ������500mLˮ�����Ƶ���Һ���������500mL����B����
C��Ӧѡ��500 mL����ƿ������500mL��Һ����Ҫ��ȡ8.0g��ˮ����ͭ����C����
D����ȡ8.0g����ͭ���500mL��Һ������500mLˮ�����Ƶ���Һ���������500mL����D����
��ѡA��
���� ���⿼��һ�����ʵ���Ũ����Һ�����ƣ��Ƚϻ���������c=$\frac{n}{V}$������Һ�����ơ����ʵ���Ũ��ע��һ��������������ƿֻ��������Ӧ�������Һ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��ѧ�� | Si-O | Si-Cl | H-H | H-Cl | Si-Si | Si-C | Cl-Cl |
| ����/kJ•mol-1 | 460 | 360 | 436 | 431 | 176 | 347 | 243 |
| A�� | �������ȶ��Ĺ��ۼ���Si-Si | |
| B�� | Cl2��g����2��Cl��g������H=-243��kJ•mol | |
| C�� | H2����g��+Cl2��g��=2HCl��g������H=-183��kJ•mol | |
| D�� | ���ݱ��������ܼ����SiCl4��g��+2��H2��g���TSi��s��+4��HCl��1���ġ�H |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �٢� | C�� | �ڢ� | D�� | �ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ϩ�Ľṹ��ʽCH2CH2 | B�� | �۱�ϩ�Ľṹ��ʽ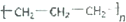 | ||
| C�� | �ǻ��Ľṹ��ʽ | D�� | 3-��-1-��ϩ�ļ���ʽ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ķϵ�ز��������Ⱦ | |
| B�� | п�̸ɵ�ظ���п�ڹ���ʱʧȥ���� | |
| C�� | ����ȼ�ϵ���ǽ�����ֱ��ת��Ϊ���� | |
| D�� | ���ε�صij������ǽ���ѧ��ת���ɵ��ܵĹ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �¶�/�� | T1 | T2 | T3 | T4 |
| ������������/g | 23.90 | 23.10 | 22.94 | 22.30 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com