
����ʯ�ǹ�ҵ��������Ҫԭ��֮һ������Ҫ�ɷ�Ϊ����������������в�����Ԫ�غ���Ԫ�أ������ʲ������ᷴӦ����ij�о���ѧϰС���ij����ʯ������������Ļ�ѧʽ����̽����
��.����ʯ�к������IJⶨ������ʵ����̲��������벹��������
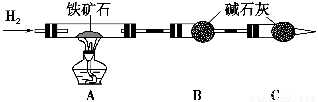
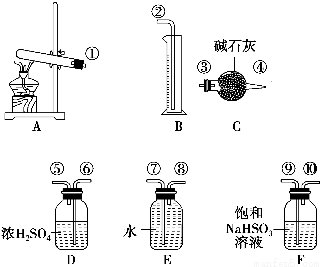
��1������ͼ��װ��������______________________________________________��
��2����8.0 g����ʯ����Ӳ�ʲ���������װ��B��C�е�ҩƷ��ͼ��ʾ���г�������ʡ�ԣ���
��3������˵����ܿڴ����ϵػ���ͨ��H2��____________________________��
��ȼA���ƾ��ƣ�
��4����ַ�Ӧ���������ƾ�����________________________________________��
��5����÷�Ӧ��װ��B����2.25 g��������ʯ�����İٷֺ���Ϊ________��
��.����ʯ�к������IJⶨ���������¡�
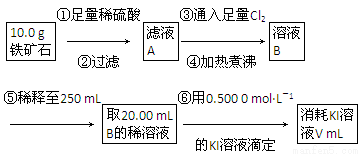
��1������������������___________________________________________��
��2�����������õ��IJ����������ձ�����ͷ�ιܡ�250 mL����ƿ��________��
��3�������йز������IJ�����˵����ȷ����________��
a����Ϊ��ˮΪ��ɫ�����Եζ������������ָʾ��
b����ƿ����Ҫ�ô���Һ��ϴ
c���ζ������п����õ�����Һ��ָʾ��
d���ζ����������۾�ע�ӵζ�����Һ��仯
e���ζ���������30 s����Һ���ָ�ԭ������ɫ���ٶ���
f���ζ����������ζ��ܼ��첿������������ⶨ���ƫ��
��4�����ζ�����������0.500 0 mol��L��1 KI��Һ20.00 mL��������ʯ�����İٷֺ���Ϊ________��
��.�����������������������ʯ������������Ļ�ѧʽΪ________��
��.��1�����װ�õ������ԡ���3����װ��C���ڴ������鴿��4���ٳ���ͨ��������Ӳ�ʲ�������ȫ��ȴ����5��25.0%
��.��1��������Һ���ܽ�Ĺ���Cl2����2������������3��be����4��70.0%
��.Fe4O5
�����������⿼������ʯ������������Ļ�ѧʽ��̽����
��.ʵ�鿪ʼǰһ��Ҫ�ȼ��װ�õ�����������װ��ҩƷ���ڵ�ȼA���ƾ���֮ǰһ��Ҫ�ȼ��������Ĵ��ȣ������ƾ��ƺ���һ��Ҫ��ͨ������һ��ʱ����ֱ��Ӳ�ʲ�������ȴ���������Է������±�������װ��B���ӵ�������Ϊ����ˮ������������m��O����2.25 g��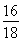 ��2.0 g����Ԫ�ص���������Ϊ
��2.0 g����Ԫ�ص���������Ϊ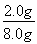 ��100%��25.0%����.��1����Ϊ����KI��Һ���еζ���������Ҳ����KI��Ӧ��Ӱ��ʵ���������Ա����ȥ��Һ���ܽ����������2������һ�����ʵ���Ũ�ȵ���Һ����Ҫ�ò��������衢��������3����ˮ�Ļ�ɫ��������a���ζ�ʱ������Ӧ��2Fe3����2I��=2Fe2����I2���ζ�ʱ����������������Һ���������ζ��յ�ʱû����ɫ�仯�����Բ����õ�����ָʾ����c���ζ�������Ҫ�۲���ƿ����Һ��ɫ�ı仯������ע�ӵζ�����Һ��仯��d���ζ����������ζ��ܼ��첿����������������KI��Һ������ƫ�����ⶨ���ƫ����f����
��100%��25.0%����.��1����Ϊ����KI��Һ���еζ���������Ҳ����KI��Ӧ��Ӱ��ʵ���������Ա����ȥ��Һ���ܽ����������2������һ�����ʵ���Ũ�ȵ���Һ����Ҫ�ò��������衢��������3����ˮ�Ļ�ɫ��������a���ζ�ʱ������Ӧ��2Fe3����2I��=2Fe2����I2���ζ�ʱ����������������Һ���������ζ��յ�ʱû����ɫ�仯�����Բ����õ�����ָʾ����c���ζ�������Ҫ�۲���ƿ����Һ��ɫ�ı仯������ע�ӵζ�����Һ��仯��d���ζ����������ζ��ܼ��첿����������������KI��Һ������ƫ�����ⶨ���ƫ����f����
��4������2Fe3����2I��=2Fe2����I2��֪10.0 g����ʯ����n��Fe����0.500 0 mol��L��1��0.02 L��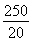 ��0.125 mol��m��Fe����7.0 g������Ԫ�ص���������Ϊ70.0%����.��������������n��Fe����n��O����
��0.125 mol��m��Fe����7.0 g������Ԫ�ص���������Ϊ70.0%����.��������������n��Fe����n��O����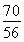 ��
�� ��4��5�������仯ѧʽΪFe4O5��
��4��5�������仯ѧʽΪFe4O5��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014����л�ѧ���ִ���ѵ���� ר��7�������Һ��ϰ���������棩 ���ͣ�ѡ����
��Na2CO3��Һ������������Ũ�ȵĹ�ϵ��ȷ���� ����������
A��c��Na������2c��CO32-�� B��c��H������c��OH����
C��c��CO32-����c��HCO3-�� D��c��OH������c��H����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014����л�ѧ���ִ���ѵ���� ר��3������������Ҫ��Ӧ������ϰ���������棩 ���ͣ�ѡ����
����˵����ȷ���� ����������
A�����ݷ�ӦCu��H2SO4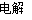 CuSO4��H2�����Ƴ�Cu�Ļ�ԭ�Ա�H2��ǿ
CuSO4��H2�����Ƴ�Cu�Ļ�ԭ�Ա�H2��ǿ
B���ڷ�ӦCaH2��2H2O=Ca��OH��2��2H2������ˮ��������
C����Ӧ3NO2��H2O=2HNO3��NO���������ͻ�ԭ�������ʵ���֮����3��1
D����Cl2��������ǿ��I2���������������û���ӦI2��2NaClO3=2NaIO3��Cl2���ܷ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014����л�ѧ���ִ���ѵ���� ר��15�л���ѧ����ѡ����ϰ���������棩 ���ͣ������
����˫ϩ�뺬��˫���Ļ��������������������Ԫ��״���������������л��ϳ������磺
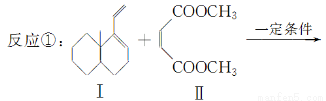
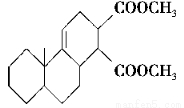
���������ɷ������·�Ӧ��
��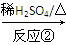 ��������ʽΪC4H4O4��
��������ʽΪC4H4O4�� 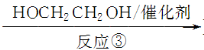 �����߷��ӻ����
�����߷��ӻ����
��1��������I�ķ���ʽΪ________��1 mol��������ȫȼ��������________mol O2��
��2����Ӧ���Ļ�ѧ����ʽΪ__________________________________________��
��3����Ӧ�������۷�Ӧ�����������Ľṹ��ʽΪ________��
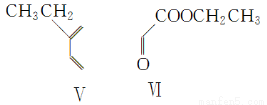
��4����һ�������£���������ͻ�������ܷ������Ʒ�Ӧ�ٵķ�Ӧ���������ֻ������Ϊͬ���칹�壩�������ֻ�����Ľṹ��ʽ�ֱ�Ϊ________��________��
��5�����й��ڻ���������������������˵����ȷ����________������ĸ����
A�������ڷ�����
B����������������ʹ������Ȼ�̼��Һ��ɫ
C����������H2�ӳɺ�IJ�����3��������
D����������������������Cu��OH��2��Ӧ���ɺ�ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014����л�ѧ���ִ���ѵ���� ר��14���ʽṹ������ѡ����ϰ���������棩 ���ͣ������
�ڵ�����������м������ʯ������A�����棩��������Al2O3�۵�����á�����ʯ������ԭ��Ϊ2Al��OH��3��12HF��3Na2CO3=2A��3CO2����9H2O�������������������գ�
��1������ʯ�Ļ�ѧʽΪ________���������Ӽ���________�Ȼ�ѧ����
��2���������к���10�����ӵķ�����________��д����ʽ�����÷��ӵĿռ乹��Ϊ________������ԭ�ӵ��ӻ���ʽΪ________��
��3����Ӧ���е縺������Ԫ��Ϊ________����Ԫ�ط��ţ���д����ԭ��������ĵ����Ų�ͼ��________��
��4��Al���ʵľ�����ԭ�ӵĶѻ���ʽ��ͼ����ʾ���侧��������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ��ͼ����ʾ��

����֪Al��ԭ�Ӱ뾶Ϊd��NA���������ӵ�������Al�����ԭ������ΪM����һ��������Alԭ�ӵ���ĿΪ________��Al������ܶ�Ϊ________������ĸ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014����л�ѧ���ִ���ѵ���� ר��13��ѧʵ���ۺ�Ӧ����ϰ���������棩 ���ͣ�ʵ����
����ͭ���ȷֽ���������ͭ�������������¶Ȳ�ͬ������ɷ�Ҳ��ͬ������ɷֿ��ܺ�SO2��SO3��O2�е�һ�֡����ֻ����֡�ij��ѧ����С��ͨ�����̽����ʵ�����ⶨ��Ӧ������SO2��SO3��O2�����ʵ�����������ȷ�������ʵĻ�ѧ���������Ӷ�ȷ��CuSO4�ֽ�Ļ�ѧ����ʽ��ʵ���õ�����������ͼ��ʾ��
[�������]
��.��������ijɷֿ���ֻ��SO3һ�֣�
��.��������ijɷֿ��ܺ���________���֣�
��.��������ijɷֿ��ܺ���________���֡�
[ʵ��̽��]
ʵ����������ԡ���֪ʵ�����ʱ������ͭ��ȫ�ֽ⡣
��1��������װ̽��ʵ���װ�������������ҵķ������������ӿڵ�����˳��Ϊ��������������������________��________��________��________��������ӿ���ţ���
��2����ʵ�����ʱB����Ͳû���ռ���ˮ����֤������________��ȷ��
��3��������ʵ��С����и�ʵ�������ڼ���ʱ���¶Ȳ�ͬ��ʵ����������������Ҳ��ͬ���������£�
ʵ�� С�� | ��ȡCuSO4 ������/g | װ��C���� ������/g | ��Ͳ��ˮ���������ɱ�״������������/mL |
һ | 6.4 | 2.56 | 448 |
�� | 6.4 | 2.56 | 224 |
��ͨ���������ƶϳ���һС��͵ڶ�С���ʵ��������CuSO4�ֽ�Ļ�ѧ����ʽ��
��һС�飺________________________________________________________��
�ڶ�С�飺________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014����л�ѧ���ִ���ѵ���� ר��12��ѧʵ�������ϰ���������棩 ���ͣ�ѡ����
ijѧ������ʵ���Ժ�����ȡ���·����ֱ���ϴ��������������Ũ��ˮ��ϴ����������Ӧ���Թܣ����þƾ���ϴ����������ʵ����ձ�������Ũ������ϴ����KMnO4�ֽ�ʵ����Թܣ�����������ϴ���ڴ�Ź�FeCl3��Һ���Լ�ƿ������NaOH��Һ��ϴʢ�����ӵ��Թܡ�����Ϊ���IJ��� ����������
A�������� B���ۢ����� C���ܢ����� D��ȫ����ȷ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014����л�ѧ���ִ���ѵ���� ר��11�����л������P��Ӧ����ϰ���������棩 ���ͣ�ѡ����
�����й��л���ѧ��Ӧ֮�������ϵ����ȷ���� ����������
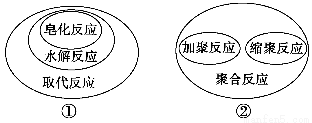
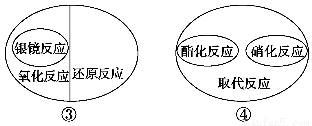
A���٢ڢ� B���٢ۢ� C���٢ڢۢ� D���٢ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���һ��ѧ�˽̰���� 4-1-2��ˮ��Դ�Ŀ���������ϰ���������棩 ���ͣ�ѡ����
����Ԫ����Cl����Na����Br����I����Mg����U�����ں�ˮ�е���Ԫ�ص��ǣ� ��
A���٢ڢ� B���ܢ�
C���٢ڢۢ� D���ۢܢݢ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com