
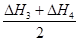 (3) ��80 kJ��mol-1
(3) ��80 kJ��mol-1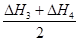 ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 CH3OH(g)+H2O(g) ��H = ��49.0 kJ��mol-1
CH3OH(g)+H2O(g) ��H = ��49.0 kJ��mol-1 CH3CH2OH(g)+3H2O(g) ��H =" ��173.6" kJ��mol-1
CH3CH2OH(g)+3H2O(g) ��H =" ��173.6" kJ��mol-1 2CH3OH���Ȼ�ѧ����ʽΪ��__________________��
2CH3OH���Ȼ�ѧ����ʽΪ��__________________��

 2CO2(g)+ N2(g) ��NO��Ũ�����¶�(T)�����������(S)��ʱ��(t)�ı仯���ߣ��������ı����S1��S2 ������ͼ�л���NO��Ũ����T1��S2 �����´ﵽƽ������еı仯���ߣ���ע��������
2CO2(g)+ N2(g) ��NO��Ũ�����¶�(T)�����������(S)��ʱ��(t)�ı仯���ߣ��������ı����S1��S2 ������ͼ�л���NO��Ũ����T1��S2 �����´ﵽƽ������еı仯���ߣ���ע���������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 H2(g)��CO(g)�� ��H����131.3 kJ����S����133.7 J/K
H2(g)��CO(g)�� ��H����131.3 kJ����S����133.7 J/K 2NH3(g)��K�䣽0.5��
2NH3(g)��K�䣽0.5�� N2(g)��3H2(g)��K��__________(����ֵ)��
N2(g)��3H2(g)��K��__________(����ֵ)���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��-69.4kJ/mol | B��-45.2 kJ/mol | C��+69.4kJ/mol | D��+45.2 kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
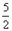 O2=2CO2(g)+H2O(l) ��H��-1300kJ/mol��˵���У���ȷ���ǣ��� ��
O2=2CO2(g)+H2O(l) ��H��-1300kJ/mol��˵���У���ȷ���ǣ��� ��| A����10NA������ת��ʱ���÷�Ӧ�ų�1300kJ������ |
| B����NA��ˮ����������ΪҺ��ʱ������1300kJ������ |
| C����2NA��̼�����õ��Ӷ�����ʱ���ų�1300kJ������ |
| D����8NA��̼�����õ��Ӷ�����ʱ���ų�1300kJ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ȷ�Ӧһ����Ҫ���Ȳ��ܷ��������ȷ�Ӧ����Ҫ����������κ��������ܷ��� |
| B�����������仯�����ʱ仯���ǻ�ѧ�仯 |
| C����C(ʯī) �� C(���ʯ)����H��+11.9 kJ/ mol��֪�����ʯ��ʯī�ȶ� |
| D����ϡ��Һ�У�H+��aq��+OH����aq����H2O��l������H����57.3 kJ/ mol��������0.5 mol H2SO4��Ũ�����뺬1 mol NaOH����Һ��ϣ��ų�����������57.3 kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A�����ѽ��γ�ȼ�� | B��¶����� |
| C����Ϊ�л����Ͻ������ϵ�ԭ�� | D��ֱ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��a��c��0 | B��b��d��0 | C��2a��b��0 | D��2c��d��0 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com