
C(s)+O2(g)![]() CO2(g) ��H2=-393.5 kJ��mol-1
CO2(g) ��H2=-393.5 kJ��mol-1
��298 KʱCO(g)��O2(g)ȼ������CO2(g)���Ȼ�ѧ��Ӧ����ʽΪ_____________________��
(2)һ�����͵�������ȼ�ϵ�ؾ��и߷���Ч�ʶ��������ӡ�����Li2CO3��Na2CO3�������λ����������ʣ�һ��ͨ��CO���壬��һ��ͨ�������CO2�Ļ�����壬�Ƶ�ȼ�ϵ�ء�
�õ�ع���ʱ�ĸ�����ӦʽΪ______________________________��
��������![]() �����ʵ����ڹ���ʱ______________(�������С�������䡱)��
�����ʵ����ڹ���ʱ______________(�������С�������䡱)��
��.����ͼ��ʾ��AΪ��Դ��BΪ������ʳ��ˮ�ͷ�̪��Һ����ֽ����ֽ�������һ��KMnO4��Һ��C��DΪ���ۣ���缫���ϼ��������Һ��ͼ��

(1)�ر�K1����K2��ͨ���B��KMnO4�Ϻ�ɫҺ����c���ƶ������Դa��Ϊ_________����ͨ��һ��ʱ��۲쵽��ֽd�˳��ֵ�������________________��

(2)��֪Cװ������ҺΪCu(NO3)2��X(NO3)3���Ҿ�Ϊ0.1 mol����K1���ر�K2��ͨ��һ��ʱ�������������������m(g)��ͨ�����ӵ����ʵ���n(mol)��ϵ��ͼ��ʾ����Cu2+��X3+��H+���������ɴ�С��˳����___________��Dװ��Cu���ĵ缫��ӦʽΪ_________��
��.(1)CO(g)+1/2O2(g)![]() CO2(g) ��H=-283.0 kJ��mol-1
CO2(g) ��H=-283.0 kJ��mol-1
����2CO(g)+O2(g)![]() 2CO2(g) ��H=-566.0 kJ��mol-1��
2CO2(g) ��H=-566.0 kJ��mol-1��
(2)CO+![]()
![]() 2CO2+2e- ����
2CO2+2e- ����
��.(1)+(����) ���
(2)Cu2+��H+��X3+ Cu-2e-![]() Cu2+
Cu2+
��������.(2)��ع���ʱ���ܷ�Ӧ����ʽΪ��
![]()
��ͨ��CO�ļ��Ǹ�����ͨ��CO2�Ϳ�����������һ���������������Ϊ���ڵ�Li2CO3��Na2CO3���ʸ�����ӦΪ��
CO+![]()
![]() 2CO2+2e-
2CO2+2e-
���ܷ�Ӧʽ֪![]() ����������������û�б仯��
����������������û�б仯��
��.(1)�˵��Ϊ���أ�ͨ���![]() ��c���ƶ���˵��c������������Դa��Ϊ������d����������������Ӧ��2H++2e-
��c���ƶ���˵��c������������Դa��Ϊ������d����������������Ӧ��2H++2e-![]() H2��������OH-��Ũ�ȱ������̪����ɫ��
H2��������OH-��Ũ�ȱ������̪����ɫ��
(2)��ͼʾ֪ת��2 mol���Ӻ����������������䣬��0.1 mol Cu2+ת��0.2 mol����ǡ��ȫ������������Cu2+��X3+��H+�����������ɴ�С��˳���ǣ�Cu2+��H+��X3+��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
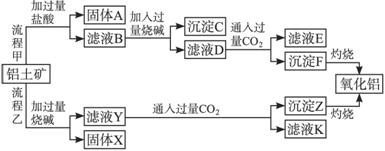
��ش��������⣺
��1�����̼������������Al3+�����ӷ���ʽΪ______________________________��
��2�������Ҽ����ռ������![]() �����ӷ���ʽΪ_____________________________��
�����ӷ���ʽΪ_____________________________��
��3����֤��ҺB��Fe3+����ȡ������Һ������_______________�����Լ����ƣ���
��4����ҺE��K�����ʵ���Ҫ�ɷ���_______________���ѧʽ����д�������ʵ�һ����;_______________��
��5����֪298 Kʱ��Mg(OH)2���ܶȻ�����Ksp=5.6��10-12��ȡ��������ҺB������һ�������ռ����ﵽ�����ܽ�ƽ�⣬���pH=13.00������¶��²�������Һ�е�c(Mg2+)=__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�갲��ʡ�������С�������ѧ�߶�3��������ѧ�Ծ����������� ���ͣ������
(4��)������������д����Ӧ���Ȼ�ѧ����ʽ��
(1)��֪16 g��������ȫȼ��ʱ�ų�148.4 kJ���������÷�Ӧȼ�յ��Ȼ�ѧ����ʽ��
____________________________________________________
(2)��ͼ��298 KʱN2��H2��Ӧ�����������仯������ͼ���÷�Ӧ���Ȼ�ѧ����ʽΪ��_________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ����10�½β��Ի�ѧ�Ծ��������棩 ���ͣ������
��10�֣���������(��Ҫ�ɷ���Al2O3����SiO2��Fe2O3��MgO������)����ȡ�����������ֹ����������£�

��ش��������⣺
��1�����̼�ͨ�����CO2�����ɳ���F�����ӷ�Ӧ����ʽΪ______________________________________��
(2�����Ҽ����ռ��ܽ�SiO2�Ļ�ѧ��Ӧ����ʽ____________________��
(3)��֤��ҺB��Fe3������ȡ������Һ������________(���Լ�����)��
(4)��ҺE��K�����ʵ���Ҫ�ɷ���________(�ѧʽ)��д�������ʵ�һ����;____________________��
(5)��֪298 Kʱ��Mg(OH)2���ܶȻ�����Ksp��5.6��10��12��ȡ��������ҺB������һ�������ռ����ﵽ�����ܽ�ƽ�⣬���pH��12������¶��²�������Һ�е�c(Mg2��)��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ר���� ���ͣ������
 2B(g) ��H=a kJ . mol-1������B�����ʵ���Ũ����ʱ��仯��ͼ��ʾ��
2B(g) ��H=a kJ . mol-1������B�����ʵ���Ũ����ʱ��仯��ͼ��ʾ�� 
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com