
����Ŀ��ij�����������ȷֽ�IJ���Ϊ������Ԫ�صĹ������ʺ�NO2��O2���壺
��1��ij�����������ȷֽ��������NO2��O2�����ʵ���֮��Ϊ6�U1�������Ԫ�صļ�̬�ڷ�Ӧ������_______(������������������������������)
��2����ȡmgCu(NO3)2��ˮ���壬ǿ��ʹ��ֽ⣬�õ�NO2��O2��ng���塣��������ˮ������պ����������ʣ�࣬ͬʱ�õ�100mL��Һ����������ɷֿ�����___________(�û�ѧʽ��ʾ)��������Һ�����ʵ���Ũ����__________________����ֻ��m�Ĵ���ʽ��ʾ����
��3����2����m = 3.76��n = 1.52����ͨ������ȷ����������ijɷ�__________________��
����Ϊ_____________________��
���𰸡� ���� CuO��Cu2O ��Cu2O 10m/92 mol/L CuO��Cu2O CuO 0.8g Cu2O 0.72g
�������������������1������NO2��O2�����ʵ���֮��Ϊ6�U1����ȷ�����ߵĻ��ϼ�Ϊ4�����͵Ļ��ϼ�Ϊ6�����ݵ�ʧ��������ȣ������Ԫ�صĻ��ϼ����ߡ�
��2����Ϊ���ɵ�������ˮ������պ����������ʣ�࣬˵�����ɵ�NO2��O2�ı�ֵ����4��1��������Ļ��ϼ�һ�����ͣ���NO2��O2�ı�ֵС��4��1������ʣ��ijɷֿ���ΪCu2O��CuO��Cu2O�Ļ�������N�غ㣬���������Һ�����ʵ���Ũ��Ϊ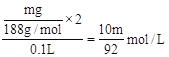 ��
��
��3��n(Cu)=![]() ���������������Ϊ1.52g��֪�����Ĺ���ΪCuO��Cu2O����CuO xmol��Cu2O ymol����
���������������Ϊ1.52g��֪�����Ĺ���ΪCuO��Cu2O����CuO xmol��Cu2O ymol����![]() �����x=0.01mol��y=0.005mol��CuOΪ0.8g��Cu2OΪ0.72g��
�����x=0.01mol��y=0.005mol��CuOΪ0.8g��Cu2OΪ0.72g��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ɫ��Һ���ܴ���������������ǣ� ��
A.Cu2+��Cl-��SO42-��K+B.Ca2+��HCO3-��Cl-��Na+
C.K+��OH-��HCO3-��Na+D.Ag+��NO3-��Cl-��K+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ڷ�Ӧ 2A2��3B2��2C ��˵�����±�ʾ�У���Ӧ���������ǣ� ��
A. v(B2)=0.8 mol/ (L��s) B. v(A2)=0.4 mol/ (L��s)
C. v(A2)=0.7 mol/ (L��s) D. v(C)=0.6 mol/ (L��s)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ӫ�����У����ܶ������ṩ�������ǣ� ��
A. ֬�� B. ˮ C. ������ D. ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2SO2(g)+O2(g) ![]() 2SO3(g)��Ӧ���̵������仯��ͼ��ʾ��
2SO3(g)��Ӧ���̵������仯��ͼ��ʾ��

��֪1mol SO2(g)����Ϊ1mol SO3(g)����H= ��99kJ��mol-1����ش��������⣺
��1��ͼ��E�Ĵ�С�Ը÷�Ӧ�ķ�Ӧ��________��������������������Ӱ�졣�÷�Ӧͨ����V2O5����������V2O5��ʹͼ��B��___________ ����������������������������
��2�����SO2����ΪSO3���Ȼ�ѧ����ʽ______________________________________��
��3����Ԫ���������л��ۿ�ʹ�������ж���1989������������֯��ʽ����ȷ��ΪʳƷ��ȾԴ֮һ�����Կ��ơ������仯���������г���ʹ��ʱ�����ϸ���Կ��Ƶ���________������ĸ��
A����������ˮ B���Ƶ��ߵ��� C���ư��ǹ��õ����� D���Ʒ�������
��4�����ڿ����лᱻ��������һ�����ܵ�����Ĥ��������������𱣻����ã����������Ĥ�����������ǿ���ǿ����ܽ⣬��д������Ĥ������������Һ��Ӧ�Ļ�ѧ����ʽ��_____________________

��5������ȱλ����ͭ��CuFe2O4-x��������������̫�����Ȼ�ѧѭ���ֽ�H2O����H2��������ת����ͼ��ʾ��д��������ȱλ����ͭ��CuFe2O4-x����ˮ��Ӧ�Ļ�ѧ����ʽ��_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���a mol N2��b mol H2�Ļ������ͨ��һ�������ݻ����ܱ������У��������·�Ӧ��N2��g��+3H2��g��2NH3��g��
��1������Ӧ���е�ijʱ��tʱ��nt��N2��=13mol��nt��NH3��=6mol������ a= ��
��2����Ӧ�ﵽƽ��ʱ�������������Ϊ716.8L������£�������NH3�ĺ��������������Ϊ25%������ƽ��ʱNH3�����ʵ��� ��
��3��ԭ���������ƽ��������������ʵ���֮�ȣ�д����������ȣ���ͬ����
n�� ʼ����n��ƽ��= ��
��4��ԭ��������У�a��b= ��
��5���ﵽƽ��ʱ��N2��H2��ת����֮�ȣ�a��N2����a��H2��= ��
��6��ƽ���������У�n��N2����n��H2����n��NH3��= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������к���ԭ����������
A. 2molCH4 B. 3molNH3 C. 4molH3PO4 D. 5molH2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ĸ���ͬ�������У��ڲ�ͬ�������½��кϳɰ���Ӧ��������������ͬʱ���ڲ�õĽ���жϣ����ɰ��ķ�Ӧ���������ǣ� ��
A.v��NH3��=0.3 mol/��Lmin��
B.v��N2��=0.01 mol/��Ls��
C.v��N2��=0.2 mol/��Lmin��
D.v��H2��=0.3 mol/��Lmin��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com