
��֪�л���ס��ҡ�����������Ϣ��
| | �� | �� | �� |
| ����Ԫ�� | C��H | C��H��F | C��H��F |
| ���������� | 26 | | |
| �ṹ�ص� | �����л��� | | |
��8�֣���1��C3H8��1�֣���4��2�֣�
��2��CH2F2��1�֣���D��2�֣�
��3��C2H5F��2�֣�
���������������1���ɱ������ݵ�֪�����DZ����л�����ķ���ʽΪC3H8��C3H8��������2����ԭ�ӱ�Fԭ��ȡ�������õ��л����������4�֡�
��2����������Ԫ��C��H��F��ԭ�Ӹ���֮��Ϊ1��2��2�����ҵĽṹ��ʽΪCH2F2��
A��Cԭ�����ӵ�Ԫ�ز�ͬ��������������ṹ���ʴ���B���ҷ�����û�в����ͼ�������ʹ��ˮ��Ӧ���ʴ���C�����к���2��Hԭ�ӣ�1 mol���������2mol F2����ȡ����Ӧ���ʴ�����ѡD��
��3����ΪC3H8����ΪCH2F2�����ס��Ұ����ʵ���֮��1��1��ϣ����û�����ƽ��Ħ���������ڱ���Ħ���������������Ӳ�����ͬ���칹�壬����ķ���ʽΪ��C2H5F��
���㣺�л������ɡ��ṹ������ ͬ���칹��
���������⿼������л������ɡ��ṹ�����ʺ�ͬ���칹���֪ʶ����Ŀ�Ѷ��У�ע��Ի���֪ʶ��ѧϰ��Ӧ�á�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015�찲��ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
��֪�л���ס��ҡ�����������Ϣ��
|
|
�� |
�� |
�� |
|
����Ԫ�� |
C��H |
C��H��F |
C��H��F |
|
���������� |
26 |
|
|
|
�ṹ�ص� |
������� |
|
|
�ݴ��ƶϣ�
��1���ķ���ʽΪ����������������2����ԭ�ӱ�Fԭ��ȡ�������õ��л�������������������֡�
��2���������������Ļ�����Ʒ���ɴ���ijЩ�ƻ�������ķ�������Ʒ������������������C��H��F��ԭ�Ӹ���֮��Ϊ1��2��2�����ҵĽṹ��ʽΪ�����������������������й����ҵ���������ȷ��������������
A. ����ӹ���Ϊ���������� B. ��������ˮ������Ӧ����ɫ
C. 1 mol���������1 mol F2����ȡ����Ӧ D. ��û��ͬ���칹
��3�����ס��Ұ����ʵ���֮��1��1��ϣ����û�����ƽ��Ħ���������ڱ���Ħ���������������Ӳ�����ͬ���칹�壬����ķ���ʽΪ��������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭���ϲ���һ���鶼��ѧ���ϲ�ʮ���и߶�5�»�ѧ���������棩 ���ͣ������
��֪�л���ס��ҡ�����������Ϣ��
|
|
�� |
�� |
�� |
|
����Ԫ�� |
C��H |
C��H��F |
C��H��F |
|
���������� |
26 |
|
|
|
�ṹ�ص� |
������� |
|
|
�ݴ��ƶϣ�
��1���ķ���ʽΪ����������������2����ԭ�ӱ�Fԭ��ȡ�������õ��л�������������������֡�
��2���������������Ļ�����Ʒ���ɴ���ijЩ�ƻ�������ķ�������Ʒ������������������C��H��F��ԭ�Ӹ���֮��Ϊ1��2��2�����ҵĽṹ��ʽΪ�����������������������й����ҵ���������ȷ��������������
A. ����ӹ���Ϊ���������� B. ��������ˮ������Ӧ����ɫ
C. 1 mol���������1 mol F2����ȡ����Ӧ D. ��û��ͬ���칹
��3�����ס��Ұ����ʵ���֮��1��1��ϣ����û�����ƽ��Ħ���������ڱ���Ħ���������������Ӳ�����ͬ���칹�壬����ķ���ʽΪ��������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��09-10������ʦ���и߶�������ĩ���Ի�ѧ�� ���ͣ������
(15��) �����廯����ס��ҡ���������Ϊͬ���칹�壬�����ʽΪC9H8O2���йص�ת����ϵ����ͼ��ʾ����֪���ס��ҡ�������������0.1 mol D��������NaHCO3��Ӧ�����ɱ�״���µ�����4.48 L��

��ʾ����
��
(1) �Ľṹ��ʽΪ ��
(2) A���ҵķ�Ӧ�������� ��Ӧ�������л�������C ��Ϊͬϵ����� ��
a��OHC��(CH2)4��COOH
b��
c�� d��
d��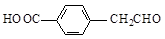
(3) д�����з�Ӧ�ķ���ʽ���л����ýṹ��ʽ��ʾ��
B���� ��
����F��Ӧ �� ��
(4)
X��E��ͬ���칹�壬X��FeCl3��Һ��ɫ������NaHCO3��Ӧ�� 1 mol X��������Na��Ӧ����1 mol H2��X�����б�����ֻ������ȡ����(���� �ṹ)����X�Ľṹ��ʽΪ
(��дһ��)��
�ṹ)����X�Ľṹ��ʽΪ
(��дһ��)��
(5)
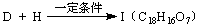 �������������I����
��
�������������I����
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�л���ס��ҡ�����������Ϣ��
| �� | �� | �� | |
| ����Ԫ�� | C��H | C��H��F | C��H��F |
| ���������� | 26 | ||
| �ṹ�ص� | �����л��� |
�ݴ��ƶϣ�
��1���ķ���ʽΪ����������������2����ԭ�ӱ�Fԭ��ȡ�������õ��л�������������������֡�
��2���������������Ļ�����Ʒ���ɴ���ijЩ�ƻ�������ķ�������Ʒ������������������C��H��F��ԭ�Ӹ���֮��Ϊ1��2��2�����ҵĽṹ��ʽΪ�����������������������й����ҵ���������ȷ��������������
A. ����ӹ���Ϊ���������� B. ��������ˮ������Ӧ����ɫ
C. 1 mol���������1 mol F2����ȡ����Ӧ D. ��û��ͬ���칹
��3�����ס��Ұ����ʵ���֮��1��1��ϣ����û�����ƽ��Ħ���������ڱ���Ħ���������������Ӳ�����ͬ���칹�壬����ķ���ʽΪ��������������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com