
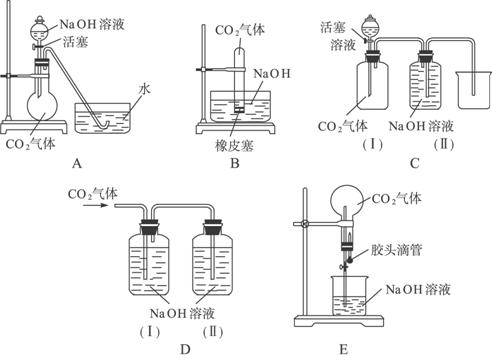
(1)ЖдЭМжаAЃЌЕБНЋЗжвКТЉЖЗжаЕФNaOHШмвКЕЮШыЩеЦПЪБЃЌШчЙћПДЕНЫЎВлжаЕФЫЎБЛЮќШыЕНЩеЦПжаЃЌдђжЄУїCO2гыNaOHШмвКЗЂЩњСЫЗДгІЁЃЧыаДГіNaOHШмвКгыЙ§СПCO2ЗДгІЕФРызгЗНГЬЪН______________________________________________________________________ЁЃ
(2)ЖдЭМBЁЊEЃЌЧыжИГіФмДяЕНЪЕбщФПЕФЕФзАжУ__________(гУзжФИЬюПе)ЃЌВЂбЁГіЦфжавЛжжЃЌЫЕУїФмжЄУїCO2гыNaOHШмвКЗЂЩњСЫЗДгІЕФВйзїМАЪЕбщЯжЯѓЃЌНЋНсЙћЬюШыЯТБэЁЃ
ЫљбЁзАжУ | ВйзїЗНЗЈ | ЪЕбщЯжЯѓ |
|
|
|
|
|
|
|
|
|
|
|
|
 УћаЃПЮЬУЯЕСаД№АИ
УћаЃПЮЬУЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКдФЖСРэНт
| ЪЕбщВйзї | ЪЕбщЯжЯѓ | ЯжЯѓНтЪЭЃЈгУРызгЗНГЬЪНБэЪОЃЉ | |
| ЬНОПЂй | AЃЎ гУВЃСЇАєеКШЁNa2S2O3ШмвКЕудкpHЪджНжаВПЃЌНЋЪджНбеЩЋгыБъзМБШЩЋПЈЖдее гУВЃСЇАєеКШЁNa2S2O3ШмвКЕудкpHЪджНжаВПЃЌНЋЪджНбеЩЋгыБъзМБШЩЋПЈЖдее |
aЃЎШмвКpH=8 | iЃЎ S2O32-+H2O?HS2O3-+OH- S2O32-+H2O?HS2O3-+OH- |
| BЃЎЯђpH=2ЕФСђЫсжаЕЮМгNa2S2O3ШмвК | bЃЎ гаЕЛЦЩЋГСЕэЃЈЛђШщАзЩЋЛызЧЃЉКЭЮоЩЋДЬМЄадЦјЮЖЦјЬхВњЩњ гаЕЛЦЩЋГСЕэЃЈЛђШщАзЩЋЛызЧЃЉКЭЮоЩЋДЬМЄадЦјЮЖЦјЬхВњЩњ |
iiЃЎS2O32вЛ+2H+ЈT SЁ§+SO2Ёќ+H2O | |
| ЬНОПЂк | CЃЎЯђаТжЦТШЫЎЃЈpHЃМ2ЃЉжаЕЮМгЩйСПNa2S2O3ШмвК | cЃЎТШЫЎбеЩЋБфЧГ | iiiЃЎ S2O32-+4C12+5H2O=2SO42-+8C1-+10H+ S2O32-+4C12+5H2O=2SO42-+8C1-+10H+ |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКдФЖСРэНт
| ЪЕбщВйзї | ЪЕбщЯжЯѓЛђдЄЦкЪЕбщЯжЯѓ | ЯжЯѓНтЪЭЃЈгУРызгЗНГЬЪНБэЪОЃЉ | |
| AЃЎ гУВЃСЇАєеКШЁNa2S2O3ШмвКЃЌЕуЕЮЕНpHЪджНЕФжабыЃЌНЋЪджНГЪЯжЕФбеЩЋгыБъзМБШЩЋПЈЖдее гУВЃСЇАєеКШЁNa2S2O3ШмвКЃЌЕуЕЮЕНpHЪджНЕФжабыЃЌНЋЪджНГЪЯжЕФбеЩЋгыБъзМБШЩЋПЈЖдее |
ШмвКpH=8 | ||
| ВТЯыЂк | ЯђpH=2ЕФСђЫсжаЕЮМгNa2S2O3ШмвК | BЃЎ гаЕЛЦЩЋГСЕэКЭЮоЩЋДЬМЄадЦјЮЖЦјЬхВњЩњ гаЕЛЦЩЋГСЕэКЭЮоЩЋДЬМЄадЦјЮЖЦјЬхВњЩњ |
S2O32-+2H+ЈTSЁ§+S02Ёќ+H2O |
| ВТЯыЂл | ЯђаТжЦТШЫЎЃЈpHЃМ2ЃЉжаЕЮМгЩйСПNa2S2O3ШмвК | ТШЫЎбеЩЋБфЧГ | CЃЎ S2O32-+4C12+5H2OЈT2SO42-+8C1-+10H+ S2O32-+4C12+5H2OЈT2SO42-+8C1-+10H+ |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК

| ||
| ||
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
БИбЁЪЕбщгУЦЗЃК

ЭъГЩЯТСаЮЪЬтЃК
(1)аДГіЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪН________________________________________________ЁЃ
(2)дкЪЕбщжаГ§ДгБИбЁЪЕбщгУЦЗжабЁдёBЁЂCЁЂDЁЂGЁЂIЭтЃЌЛЙБиаыбЁдёЕФгУЦЗЪЧ(ЬюБрКХ)______________ЁЃ
(3)дкЯТЭМЗНПђжаВЙЛГіЫљЩшМЦЪЕбщЕФзАжУЭМЃЌВЂзЂУїгаЙивЧЦїРяЪдМСЕФУћГЦЁЃ
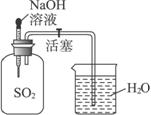
(4)МђвЊЫЕУїЪЕбщВйзїМАЙлВьЕНЕФЯжЯѓЃК_____________________ ЁЃ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com