
������Ԫ��A��B��C��D��E��F����Ԫ�أ����ǵ�ԭ��������A��F�������������ڱ��У�A��ԭ�Ӱ뾶��С��BԪ�ص�ԭ���������������ڲ��������������CΪ�ؿ��к�������Ԫ�أ�D��ԭ�Ӱ뾶���Ķ���������Ԫ�أ�D����ȼ��ʱ���ֻ�ɫ���棬D�ĵ����ڸ�������C�ĵ��ʳ�ַ�Ӧ�����Եõ���E������ɫ��ͬ�ĵ���ɫ��̬�����D��F�γɵ����ӻ�����DF�dz��õĵ�ζƷ���Ը������������ش�
��1��Ԫ�����ƣ�
A________��B________��C________��D________��
��2��E��Ԫ�����ڱ��е�λ�ã�_________________________________________________��
��3��F���ӽṹʾ��ͼ��_____________________________________________________��
��4��A��B��ɵ���������������____________________________________________��
��5��C��D��ԭ�Ӹ�����1��1��ɵ�һ�ֻ�������ˮ������Ӧ�Ļ�ѧ����ʽΪ___________________________________________________________��
��6����˵��E�ķǽ����Ա�F�ķǽ�����____���ǿ��������������ʵ��________����һ������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��û�ѧ����ش��������⣺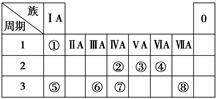
��1���ܡ��ݡ���ԭ�Ӱ뾶�ɴ�С��˳����_________________________________________��
��2���ڡ��ۡ��ߵ���ۺ������������ǿ������˳����________________________________��
��3���١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����ĵ���ʽ��___________________________________________________________��
��4���ɱ�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬�������ϡ��Һ�ױ����ֽ⣬��ʹ�õĴ���Ϊ������ţ�________��
a��MnO2 b��FeCl3 c��Na2SO3 d��KMnO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��CΪ������Ԫ�أ������ڱ���������λ����ͼ��ʾ��A��C��Ԫ�ص�ԭ�Ӻ��������֮�͵���Bԭ�ӵ���������Bԭ�Ӻ�������������������ȡ�
(1)д��A��B��C��Ԫ������______��______��______��
(2)C��Ԫ�����ڱ��е�λ����_____________________________________��
(3)B��ԭ�ӽṹʾ��ͼΪ________��C���⻯����B���⻯����ȶ���ǿ��˳��Ϊ________��________(�ѧʽ)��
(4)�Ƚ�A��C��ԭ�Ӱ뾶��A________C��д��A����̬��
������A������������Ӧˮ���ﷴӦ�Ļ�ѧ����ʽ��___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��Ԫ�����ڱ�ǰ�������е�4��Ԫ�أ�A����D���AD2�ͻ����AD2�к������������30��D���ĺ�������Ų���Al3����ͬ��B��C�����γ�BC�ͻ����BC����������������18��BCˮ��Һ��һ��ǿ�ᣬ�Իش�
��1��д����������Ԫ�ص����ƣ�A__________��B__________��C__________��D__________��
��2���õ���ʽ��ʾAD2��___________________________________ _____________________________________��
��3��D������ˮ��Ӧ�Ļ�ѧ����ʽΪ_________________________ __________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��H��D��T����ԭ�ӣ����Կ��γ�˫ԭ�ӷ���H2��D2��T2������д���и��⡣
��1����״���£����ǵ��ܶ�֮��Ϊ________��
��2����ͬ���ʵ��������ֵ����У�������֮��Ϊ________��
��3����1 g���ֵ����У����ǵ�������֮��Ϊ________��
��4��ͬ��ͬѹ�£�1 L����������������������֮��Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E��F��Ϊ����������Ԫ�أ���ԭ������������������Ԫ����C��ԭ�Ӱ뾶���B��Eͬ�壬E�������������ǵ��Ӳ�����2����A�����������ˮ����ΪH2AO3��D�ǵؿ��к�����ߵĽ���Ԫ�ء�
(1)A��Ԫ�ط�����________��C2B2�ĵ���ʽΪ________��
(2)B��C��D��F�����Ӱ뾶�ɴ�С��˳����________(�����ӷ��ű�ʾ)��
(3)E��F��ȣ��ǽ�����ǿ����________(��Ԫ�ط��ű�ʾ)��������ʵ��֤����һ���۵���________(�÷������)��
�ٳ�����E�ĵ��ʳʹ�̬��F�ĵ��ʳ���̬������̬�⻯����ȶ��ԣ�F>E����E��F�γɵĻ������У�E�����ۡ���F��������E���⻯����û���Ӧ����E��F���������ˮ���������ǿ��������̬�⻯��Ļ�ԭ�ԣ�E>F
(4)��CDB2��Һ�������õĹ�������Ϊ________(�ѧʽ)��
(5)C��F����ɻ�����ף��ö��Ե缫����ˮ��Һ�����Ļ�ѧ����ʽΪ__________________________��
(6)A��B��C����Ԫ����ɵij����������ҵ���Һ�У�����Ũ���ɴ�С��˳��Ϊ________��pH��10������Һ����ˮ���������c(OH��)��________mol��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ԫ�ص�ԭ��������11����Ԫ��ԭ�Ӻ������������Ӳ㣬������Ӳ�����6�����ӣ�����Ԫ�����ڱ��ڢ�A���н����������ļ����Ԫ�ء��ɴ��ƶϣ�
��1����Ԫ�������ڱ���λ�ڵ�________���ڣ���Ԫ�������ڱ���λ�ڵ�________�壻��Ԫ�ص�����Ϊ________��
��2���ĵ�����ˮ��Ӧ�����ӷ���ʽΪ__________________________���ҵ���������ʷ�Ӧ�Ļ�ѧ����ʽΪ______________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
2009�꡶��Ȼ����־�������ҹ���ѧ��ͨ������SiO2��26Al��10Be����Ԫ�صı���ȷ���������ˡ�������о���������ֲ��������С�������귨�������������գ�
��1��10Be��9Be________��
a����ͬһ��ԭ��
b��������ͬ��������
c��������ͬ�Ļ�ѧ����
��2��Al��Be�������ƵĻ�ѧ���ʣ�д��BeCl2ˮ�ⷴӦ�Ļ�ѧ����ʽ_______________��
��3���о�����26Al����˥��Ϊ26Mg�����ԱȽ�������Ԫ�ؽ�����ǿ���ķ�����_________
_______________________________________________________________��
a���Ƚ�������Ԫ�صĵ��ʵ�Ӳ�Ⱥ��۵�
b�����Ȼ������Ȼ�þ����Һ�зֱ�μӹ���������������Һ
c������ĥ����þ������Ƭ�ֱ����ˮ���ã��������̪��Һ
d���������з����Ѿõ�������Ԫ�صĵ��ʷֱ����ˮ����
��4��Ŀǰ����һ�ֲ��������С���벲��귨����д����Ar��������Ų���ͬ�������ӵİ뾶�ɴ�С��˳��________(�û�ѧ���ű�ʾ)������һ�������������Ԫ�ص��������γɵĻ������������������˻�����ĵ���ʽ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Ԫ��X��Y��Z��W��ԭ��������������X���⻯���ˮ��Һ�Լ��ԣ�Y��Ԫ�����ڱ�����������������������������ȣ�Z�ĵ����ǽ�̫����ת��Ϊ���ܵij��ò��ϣ�W����Ҫ�ġ�����Ԫ�ء�����Ҫ�����ε���ʽ�����ں�ˮ�С�
��ش��������⣺
(1)Y��Ԫ�����ڱ��е�λ����________��X���⻯��ĵ���ʽ��________��
(2)X���⻯���ˮ��Һ��W���⻯���ˮ��Һ��Ϻ�ǡ�÷�Ӧʱ����Һ��________(��ᡱ��������С�)�ԣ������ӷ���ʽ��ʾ��ԭ��____________________��
(3)Z��W�Ƚϣ��ǽ����Խ�������________(��Ԫ�ط���)�����п�����֤��һ���۵���________(�����)��
a��Ԫ���ڵؿ��еĺ���
b���������Ӧˮ���������
c���Ͽ��⻯����1 mol H��Z��H��W�����������
d��Z��W�Թ��ۼ��γɻ�����ʱ��Z��W��ʾ�ĵ���
(4)��Y����������Һ��ϲ�����ɫ������д����Ӧ�����ӷ���ʽ��______________________________________________________��
(5)X��Z��W������������Ӧ��ˮ���������ǿ��˳��Ϊ________(�û�ѧʽ��ʾ����ͬ)��Z��W�ļ���̬�⻯��Ļ�ԭ��ǿ��˳��Ϊ________��X��Z��W������⻯���У��е���ߵ���________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com