
��13�֣���ij�о���ѧϰС��Ϊ��̽������ĵ������������������ʵ�飮
��1�������Ʋ��궨������Һ��Ũ��
ȡ����������250 mL 0.2 mol/L�Ĵ�����Һ����0.2 mol/L�Ĵ�����Һϡ�ͳ�����Ũ�ȵ���Һ������NaOH����Һ�����������Һ��Ũ�Ƚ��б궨���ش��������⡣
������250 mL 0.2 mol/L������Һʱ��Ҫ�õ��IJ�����������Ͳ���ձ�����ͷ�ιܡ���������________��
��Ϊ�궨ij������Һ��ȷŨ�ȣ���0.200 0 mol/L��NaOH��Һ��20.00 mL������Һ���еζ������в����п���ʹ���������Һ��Ũ����ֵƫ�͵���________��
| A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע�����Һ |
| B���ζ�ǰ��ƿ������ˮϴ����û�и��� |
| C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ |
| D����ȡ�����������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��� |
250 mL����ƿ��AD > �ޡ�>
�������������
��1����A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע�����Һ������Ũ�Ƚ��ͣ���������������Һ�����С������������ҺŨ��ƫ�͡�
B���ζ�ǰ��ƿ������ˮϴ����û�и����Ӱ��������ҺŨ�ȡ�
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ����������������Һ���������������ҺŨ��ƫ�ߡ�
D����ȡ�����������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���������ֵС��ʵ��ֵ��������ҺŨ��ƫС��
��ѡAD��
��2����Ϊ������������ʣ����ֵ��룬��ˮϡ��10��pH�仯С��1�����Լ�ˮ����Һ���V�����ᣩ��V�����ᣩ��
��3������������ˮ�ĵ��룬ֻ�Т�ʵ��Ϊ�Ȼ�����Һ����ˮ�ĵ��������ơ���ΪpH��12�İ�ˮ��c(NH3��H2O)��0.01mol/L���ʽ��ڡ��ۻ�Ϻ�������ҺpH��7����������Һ��������ڣ��ۡ�
���㣺��Һ�����ƣ��к͵ζ���������ʵĵ��롣
�����������ۺ���ǿ����ѧ��֪ʶ��ȫ����Ҫ��ϸߡ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
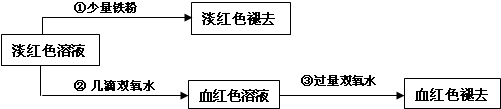
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
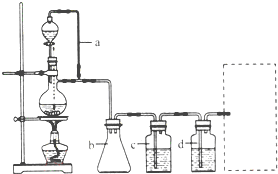 ��2011?���ij�о���ѧϰС��Ϊ�ϳ�1-�������������ϵ�֪һ���ϳ�·�ߣ�
��2011?���ij�о���ѧϰС��Ϊ�ϳ�1-�������������ϵ�֪һ���ϳ�·�ߣ�| һ������ |
| ||
| Ni���� |
| ||
| �� |
| ���� |
| ���� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
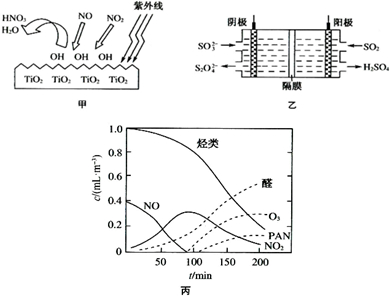
| O | 2- 4 |
| O | 2- 3 |
| O | 2- 4 |
| O | 2- 4 |
| O | 2- 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com