
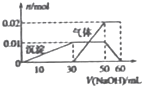
 ij��ɫ��Һ��ֻ����NH4+��K+ Al3+ Cu2+��Mg2+��CO32-��SO42-�������еļ��֣���1��ȡ100mL����Һ���μ�����ϡ�����ữ��Ba��NO3��2��Һ�����˵õ�0.03mol��ɫ����
ij��ɫ��Һ��ֻ����NH4+��K+ Al3+ Cu2+��Mg2+��CO32-��SO42-�������еļ��֣���1��ȡ100mL����Һ���μ�����ϡ�����ữ��Ba��NO3��2��Һ�����˵õ�0.03mol��ɫ����| A����Һ��һ��������Cu2+��Mg2+��CO32-���ܺ���K+ | B��NH4+��Al3+��SO42-�������ӵ����ʵ���֮��Ϊ2��1��3 | C��ʵ�������õ�NaOH��Һ�����ʵ���Ũ��Ϊ0.1mol/L | D��ֻ��ͨ����ɫ��Ӧʵ����ȷ�ϸ���Һ���Ƿ����K+ |
| n |
| V |
| 0.06mol |
| 0.06L |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� | ʵ�������� | |
| ����1 | ȡ������ɫ��Һ������NaOH | �������ɺ��ɫ������˵����ˮ��FeSO4��Һ�����˻�ѧ��Ӧ |
| ����2 | ȡ������ɫ��Һ��������۵⻯����Һ | ��Һ����ɫ��˵��δ������ѧ��Ӧ |
| ����3 | ȡ������ɫ��Һ���������Ȼ�̼���� | �²���Һ�ʳȺ�ɫ��˵��δ������ѧ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ij��Һ�еμ�BaCl2��Һ�ð�ɫ������ȡ�ð�ɫ������ϡ����ܽ�--ԭδ֪��Һ��һ������SO42- | B����ijδ֪��Һ�м���Ũ��NaOH��Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ����������--ԭδ֪��Һ��һ������NH4+ | C����ijδ֪��Һ�еμ����������ɫ���壬��������ͨ����������ʯ��ˮ�еð�ɫ����--ԭδ֪��Һ��һ������CO32- | D���ýྻ�IJ�˿պȡδ֪���ʣ�������ɫ���������գ�����ɫΪ��ɫ--��δ֪����һ��ֻ����Ԫ�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com