
ij��ѧ����С���о��ϳɰ���N2(g)+3H2(g)  2NH3(g)����H<0,��������������ʱ���ı�ijһ����ʱ�Ի�ѧƽ���Ӱ�죬�õ�����ͼ�����¶�Ӧѡ������ȷ���ǣ� ��
2NH3(g)����H<0,��������������ʱ���ı�ijһ����ʱ�Ի�ѧƽ���Ӱ�죬�õ�����ͼ�����¶�Ӧѡ������ȷ���ǣ� ��
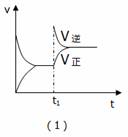

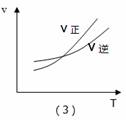
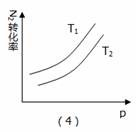
A.��1����Ӧ���ǣ���t1ʱ��ѹ�����£�ͨ��NH3
B. ��2����Ӧ���ǣ������ں��ݲ�ͬ�¶��µİٷֺ���
C.��3����Ӧ���ǣ��ں��������£���Ӧ�������¶ȵĹ�ϵ
D. ��4����Ӧ���ǣ�N2ת�������¶ȣ�T1>T2����ѹǿ�Ĺ�ϵ
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ����������ʱ�����ô�������Ӧ��SO2 ת��ΪSO3��һ���ؼ����衣ѹǿ���¶ȶ�SO2ת���ʵ�Ӱ�����±���ԭ�������ɷֵ��������Ϊ�� SO2 7% ��O2 11%��N2 82%����
SO2 7% ��O2 11%��N2 82%����
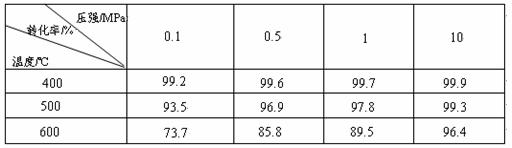
��1����֪SO2�������Ƿ��ȷ�Ӧ��������ñ��������ƶϴ˽��ۣ�
��
��2���ڴ�400��500��ʱ��SO2�Ĵ��������ó�ѹ�����Ǹ�ѹ����Ҫԭ���ǣ�
��
��3��ѡ�����˵Ĵ������Ƿ�������SO2��ת���ʣ� ����ǡ������Ƿ��������÷�Ӧ���ų��������� ����ǡ�����
��4��Ϊ���SO3�����ʣ�ʵ���������� ����SO3��
��5����֪��2SO2(g)+O2(g)��2SO3(g)����H����196.6kJ��mol��1������ÿ����1���98%��������Ҫ��SO3��������SO2������ЩSO3���ų���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D��E�������ʻ������֮����ת����ϵ����ͼ��ʾ��AΪ�ؿ��к������������ķǽ���Ԫ�صĵ��ʣ��侧��ṹ����ʯ���ơ�
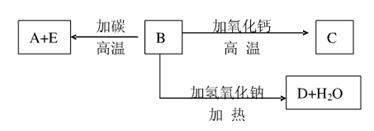
����գ�
�γɵ���A��ԭ�ӵĽṹʾ��ͼΪ_________________,��������ϼ�Ϊ______________��
B�Ļ�ѧʽ������ʽ��Ϊ_______________��B�ľ�������Ϊ___________��B��̼��Ӧ����A��E�Ļ�ѧ����ʽ��_________��
C�Ļ�ѧʽ������ʽ��Ϊ___________��D�Ļ�ѧʽ������ʽ��Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��λ�����ϡ��Һ�У��ǻӷ������ʵķ��ӻ�������Խ�࣬����Һ�ķе��Խ�ߣ���������Һ�е���ߵ���(����)
A��0.01 mol/L��������Һ B��0.01 mol/L��CaCl2��Һ
C��0.02 mol/L��NaCl��Һ D��0.02 mol/L��CH3COOH��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
NaNO2��һ��ʳƷ���Ӽ��������°�������KMnO4��Һ��NaNO2��Ӧ�����ӷ���ʽΪMnO4- + NO2- + H+ — Mn2+ + �� + H2O ��δ��ƽ��. ����������ȷ���ǣ� ��
A ��Ӧ����ҺpH�½� Bͨ���������ữ�ĸ��������Һ
C ����������ΪNO2 D. ������1 mol Mn2+ ������2.5 mol NO2-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ���ǣ� ��
A. ͬһ����Ԫ����̬�⻯����ϵ��£���е�������
B���ǽ���������һ�����ڹ��ۼ������ӻ������п��ܴ��ڹ��ۼ�
C.�Ǽ��Լ������ܴ��������ӻ������У��ɷǽ���Ԫ����ɵĻ�������һ�����������Ӽ�
D.���������ӵ�����һ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����SO2�������Ƿ����CO2���壬�ɲ��õķ�����
A��ͨ��Ʒ����Һ
B��ͨ�� ����ʯ��ˮ
����ʯ��ˮ
C����ͨ��NaOH��Һ����ͨ������ʯ��ˮ
D����ͨ������KMnO4��Һ����ͨ������ʯ��ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʵ������ȡSO2����֤SO2��ijЩ���ʵ�װ��ͼ���Իش��������⡣

(1)�ڢ��з����Ļ�ѧ��Ӧ����ʽΪ_________________________________��
(2)���е�ʵ������Ϊʯ����Һ________����ʵ��֤��SO2��ˮ��Ӧ���ɲ����________�ԡ�
(3)���е�Ʒ����Һ________��֤��SO2��________��
(4)���е�ʵ��������___________________________________________��֤��SO2��________�ԡ�
(5)���е�ʵ��������___________________________________________________________��
֤��SO2��________�ԡ�
(6)�ݵ�������______________________________________________________________��
��Ӧ�Ļ�ѧ����ʽΪ________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���г����µ��ķ���Һ����0.01 mol/L CH3COOH����0.01 mol/L HCl����pH=12�İ�ˮ����pH=12��NaOH��Һ������˵����ȷ����
A.����ˮ�ĵ���̶���С
B.���١��ܻ�ϣ���c��CH3COO-��>c��OH-������Һһ���ʼ���
C.ϡ����ͬ��������Һ��pH����>��
D.���ڡ��ۻ�ϣ���pH=7����������Һ���������>��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com