
| ʱ��/min | 0 | 5 | 10 | 15 | 20 | 25 | 30 |
| c��X��/mol•L-1 | 0.2 | c | 0.6 | 0.6 | 1.0 | c1 | c1 |
| c��YX��/mol•L-1 | 0.6 | c | 0.4 | 0.4 | 0.4 | c2 | c2 |
���� ��1��N2H4���H2O�����������ʹ��Һ�ʼ��ԣ�
��2����Ӧ��=��Ӧ���ܼ���-�������ܼ��ܣ�
��3����X��Y����ʼŨ�ȷֱ�Ϊ0.2mol/L��0.6mol/L��ǰ10min�ڣ�XŨ��������0.4mol/L��Y��Ũ�ȼ�С0.2mol/L������NO2��N2O4�밴���ʵ���2��1��Ӧ����XΪNO2��YΪN2O4��ƽ�ⳣ��K=$\frac{{c}^{2}��N{O}_{2}��}{c��{N}_{2}{O}_{4}��}$���������ʵ�Ũ�ȼ��㣻
�ڸ���v=$\frac{��c}{��t}$����v��NO2����20minʱ��Y��N2O4����Ũ�Ȳ��䣬X��NO2����Ũ�����ı������Ӧ������NO2��Ũ�ȣ���ЧΪ����ѹǿ��ƽ��������N2O4�����淴Ӧ���У�����2molNO2��ͬʱ������������ʵ�����С1mol������������NO2�İٷֺ�����С��
��4������0.2mo1•L-1N2H4•H2O��Һ��0.1mol•L-1HCl��Һ�������ϣ��õ����ʵ�Ũ�����N2H5C1��N2H4•H2O��������ͬ������N2H4•H2O�ĵ���̶ȴ���N2H5C1��ˮ��̶ȣ���������Ũ�ȴ�СΪ��c��N2H5+����c��Cl-����c��OH-����c��H+�����ɴ˽��
��� �⣺��1����������ˮ�������백���Ƶ�������������OH-�������ӣ����뷽��ʽΪN2H4+H2O?N2H+5+OH-��
�ʴ�Ϊ��N2H4+H2O?N2H+5+OH-��
��2����1mol N2O4��1����ȫ�ֽ����Ӧ��ԭ��ʱ��Ҫ���յ�����QKJ����
190kJ/mol��2+390kJ/mol��8+QkJ/mol-946kJ/mol��3-460kJ/mol��8=-1225 kJ/mol��
���Q=1793��
�ʴ�Ϊ��1793kJ��
��3����X��Y����ʼŨ�ȷֱ�Ϊ0.2mol/L��0.6mol/L��10minʱ����ƽ�⣬XŨ��������0.4mol/L��Y��Ũ�ȼ�С0.2mol/L������NO2��N2O4�밴���ʵ���2��1��Ӧ����XΪNO2��YΪN2O4��
ƽ�ⳣ��K=$\frac{{c}^{2}��N{O}_{2}��}{c��{N}_{2}{O}_{4}��}$=$\frac{0��{6}^{2}}{0.4}$=0.9��
�ʴ�Ϊ��NO2��0.9��
��v��NO2��=$\frac{0.6mol/L-0.2mol/L}{10min}$=0.04mol/��L��min����20minʱ��Y��N2O4����Ũ�Ȳ��䣬X��NO2����Ũ�����ı������Ӧ������NO2��Ũ�ȣ���ЧΪ����ѹǿ��ƽ��������N2O4�����淴Ӧ���У�����2molNO2��ͬʱ������������ʵ�����С1mol������������NO2�İٷֺ�����С��
�ʴ�Ϊ��0.04mol/��L��min��������NO2��Ũ�ȣ�b��
��4������0.2mo1•L-1N2H4•H2O��Һ��0.1mol•L-1HCl��Һ�������ϣ��õ����ʵ�Ũ�����N2H5C1��N2H4•H2O��������ͬ������N2H4•H2O�ĵ���̶ȴ���N2H5C1��ˮ��̶ȣ���������Ũ�ȴ�СΪ��c��N2H5+����c��Cl-����c��OH-����c��H+�����ʴ�Ϊ��c��N2H5+����c��Cl-����c��OH-����c��H+����
���� ���⿼�黯ѧƽ���йؼ��㡢��ѧƽ��Ӱ���ƶ�����Ӧ�ȼ���ȣ��Ƕ�ѧ���ۺ������Ŀ��飬�Ѷ��еȣ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
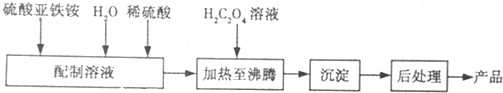
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ԭ�Ӱ뾶��C��D��A��B | B�� | ԭ��������b��a��c��d | ||
| C�� | ���Ӱ뾶��Cn+��D��n+��-��An+��B��n+��+ | D�� | ���ʻ�ԭ�ԣ�A��B��C��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ԭ��Ӧ�У�������7.1g Cl2����ת��0.2NA������ | |
| B�� | ��1.0molFeCl3ȫ���Ƴɽ��壬����������������ΪNA�� | |
| C�� | 12g NaHSO4������ʱ��������Ϊ0.3NA | |
| D�� | ��״���£�11.2L����������ԭ����Ϊ0.5NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
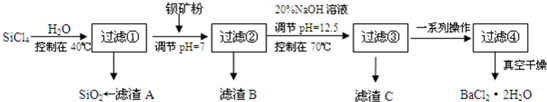
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1mol ���������������Ӹ���Ϊ2��6.02��1023 | |
| B�� | 14g��ϩ�ͱ�ϩ�Ļ�����к��е�̼ԭ�ӵ���ĿΪ6.02��1023 | |
| C�� | 28g C16O��28g C18O�к��е���������Ϊ14��6.02��1023 | |
| D�� | ��״���£�22.4L��������������������Һ��Ӧת�Ƶĵ�����Ϊ2��6.02��1023 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
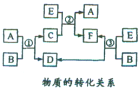 A��B��C��D��E��F����������һ������������ͼ��ʾ��ת����ϵ�����з�Ӧ�����������Ѹ�����
A��B��C��D��E��F����������һ������������ͼ��ʾ��ת����ϵ�����з�Ӧ�����������Ѹ�����| E���������״���� | 2.8L | 5.6L | 11.2L |
| n��F�������ӣ�/mol | 1.25 | 1.5 | 2 |
| n��C�е������ӣ�/mol | 1.5 | 1.4 | y |
| n��B�е������ӣ�/mol | x | 0 | 0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NH3��H2��CH4 | B�� | PCl5��CO2��H2SO4 | C�� | SO2��SiO2��P2O5 | D�� | CCl4��Na2S��H2O2 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com