
(14��)A��B��C��D������ѧ��ѧ�������ʻ����ӣ�����A��B��C������ͬһ��Ԫ�أ���һ���������ת���Ĺ�ϵ����ͼ��ʾ(���ַ�Ӧ�е�H2O����ȥ���������ﶼ���г�)���밴Ҫ��ش��������⣺
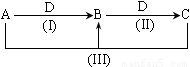
(1)��DΪ�������ʣ���D�����������;���Ľ���������������B����Һû�еõ�B���Σ���B�Ļ�ѧʽ����Ϊ_____________________����4����D���ᣬ��һ���Ƕ�Ԫ�ᣬ����ABCD �ֱ���
(2)��A�������������B��DΪ��������Ҫ�ɷ֣���Ӧ(III)�Ļ�ѧ����ʽΪ__________��
(3)��DΪ�ȼҵ����Ҫ��Ʒ����Ӧ(III)�����ӷ���ʽΪ____________________________��
(4)��DΪ����������壬Ҳ��ʵ�������ת�����밴A��B��C��D˳��д������������һ�����ʵĻ�ѧʽ��
�ٵ�DΪ��ʱ��A________��B_________��C_________��D__________��
�ڵ�DΪ��������ʱ��A________��B_________��C_________��D__________��
(1)FeCl3 ��2�֣�
(2)4NH3+6NO 5N2+6H2O��2�֣�
5N2+6H2O��2�֣�
(3)Al3++3AlO2-+6H2O==4Al(OH)3����2�֣�
(4)��Ba(OH)2��Ba3(PO4)2��BaHPO4��Ba(H2PO4)2 ��H3PO4 ��4�֣�
��NaOH��Na2CO3��NaHCO3��CO2(���������𰸸���)(4��)
����������1�����������;���Ľ�����������D�����������л�ԭ�������ڱ�۽�����A��B��C������ͬһ��Ԫ�أ���������B����Һû�еõ�B���Σ�˵�������������Σ���A��B��C���е�ͬһ��Ԫ������Ԫ�ء���ABC�ֱ����������Ȼ������Ȼ�������
��2��B��DΪ��������Ҫ�ɷ֣������Ϊ�����͵�����A��������������ǰ�����������������N2��N2��������NO��
��3��DΪ�ȼҵ����Ҫ��Ʒ��D���������ơ�ABC�������Ļ�����֮���ת������A�����Σ�B������������C��ƫ�����Ρ�
��4����D���ᣬ��һ���Ƕ�Ԫ�ᣬ����ABCD �ֱ���Ba(OH)2��Ba3(PO4)2��BaHPO4��Ba(H2PO4)2 ��H3PO4 ����DΪ��������ʱ��ҲӦ���Ƕ�Ԫ���Ӧ�����������������̼�����⡢��������ȡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2009-2010ѧ��������ѧ�߶���ѧ����ĩ���Ի�ѧ ���ͣ������
(14��)A��B��C��DΪ���ֵ��ʣ�����ʱ��A��B�����壬C��D�ǹ��塣E��F��G��H��IΪ���ֻ����F����ˮ��EΪ�����Ҽ�����ˮ��Ϊ��ɫ��Һ��G��ˮ�û���ɫ��Һ����������ʼ䷴Ӧ��ת����ϵ��ͼ��ʾ
��1��д�����ֵ��ʵĻ�ѧʽ
A____ _____ B__________ C__ ____ D___ _____
��2��д��E��F��H��I�����ӷ���ʽ
��3��д��G+I��H+D+E�Ļ�ѧ����ʽ
��4��ij������B��Ư�ۡ�
��д����Ư�۵Ļ�ѧ����ʽ ��
��Ϊ�ⶨ�ù����Ƶõ�Ư������Ч�ɷֵĺ�����ij��С�����������ʵ�飺��ȡƯ��2.0g����ĥ���ܽ⣬���ó�250mL��Һ��ȡ��25.00mL���뵽��ƿ�У��ټ��������KI��Һ���������ᣨ��ʱ���������ӷ���ʽΪ�� �������á�����ȫ��Ӧ����0.1mol��L-1��Na2S2O3��Һ������Һ�ζ���Ӧ���ɵĵ⣬��֪��ӦʽΪ��2Na2S2O3+I2=Na2S4O6+2NaI������ȥNa2S2O3��Һ20.00mL�����Ư������Ч�ɷֵ���������Ϊ ��������С�������λ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�����ʡ����һ�и���������ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ������
(14��A��B��C�� D ��E��F�dz��������壬����A��B��C��DΪ���ʣ��йص�ת����ϵ����ͼ��ʾ(��Ӧ����������ȥ����
D ��E��F�dz��������壬����A��B��C��DΪ���ʣ��йص�ת����ϵ����ͼ��ʾ(��Ӧ����������ȥ����
��ش��������⣺
(1)D�ĵ���ʽΪ ��
(2)��Ӧ �۵����ӷ���ʽΪ ��
�۵����ӷ���ʽΪ ��
(3)Y��E��һ�������¿ɷ�Ӧ����B��Z��������E�Ի�������Ⱦ���÷�Ӧ�Ļ�ѧ����ʽ Ϊ ��
Ϊ ��
(4)������0.1mol/L��Y��Һ��c(H+)/c(OH-)=1��10-8������������ȷ���� ��
�ٸ���Һ��pH=11
�ڸ���Һ�е����ʵ������������Ũ��Ϊ0.1mol/L
�۽�pH=11��Y��Һ��ˮϡ��100����pHֵΪ9
�ܸ� ��Һ��ˮ�������c(H+)��c(OH-)�˻�Ϊ1��10-22
��Һ��ˮ�������c(H+)��c(OH-)�˻�Ϊ1��10-22
��0.1mol/L��������ҺV1 L���0.1mol/L��Y��ҺV2 L��ϣ��������ҺpH=7����:V1>V2
(5)������pH=a��X��Һ��pH=b��Y��Һ�������ϣ���a+b=14�����Ϻ����Һ��________�ԣ������Һ�и�����Ũ�ȴ�С��ϵΪ______ ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�����ʡ����������ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ������
(14��A��B��C��D ��E��F�dz��������壬����A��B��C��DΪ���ʣ��йص�ת����ϵ����ͼ��ʾ(��Ӧ����������ȥ����

��ش��������⣺
(1)D�ĵ���ʽΪ ��
(2)��Ӧ�۵����ӷ���ʽΪ ��
(3)Y��E��һ�������¿ɷ�Ӧ����B��Z��������E�Ի�������Ⱦ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
(4)������0.1mol/L��Y��Һ��c(H+)/c(OH-)=1��10-8������������ȷ���� ��
�ٸ���Һ��pH=11
�ڸ���Һ�е����ʵ������������Ũ��Ϊ0.1mol/L
�۽�pH=11��Y��Һ��ˮϡ��100����pHֵΪ9
�ܸ���Һ��ˮ�������c(H+)��c(OH-)�˻�Ϊ1��10-22
��0.1mol/L��������ҺV1 L���0.1mol/L��Y��ҺV2 L��ϣ��������ҺpH=7����:V1>V2
(5)������pH=a��X��Һ��pH=b��Y��Һ�������ϣ���a+b=14�����Ϻ����Һ��________�ԣ������Һ�и�����Ũ�ȴ�С��ϵΪ______ ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�����������ѧ����1���¿��Ի�ѧ�Ծ� ���ͣ������
(14��) ��15�֣�A��B��C��D�Ƕ�����Ԫ���γɵ��������嵥�ʣ��ס��ҡ��������ǻ�������л������������Ӿ��壬DԪ�ص�ԭ�������������Ǵ�����3����C�������Ա�Dǿ�����ǵ�ת����ϵ��ͼ��ʾ�����ǵ����������������Ӧ������ȥ����

��1��д������ʽ��A ��B ��
C ��D ��
��2��д���ҵ�������ˮ������ӷ���ʽ�� ��
��3��д����Ӧ�١��ڻ�ѧ����ʽ���� ���� ��
��4���ٳ�ʵ��˵������C��D������ǿ���û�ѧ����ʽ��ʾ���� ��
��5��A��C\��D��B��Ӧ���ɵ��⻯��ķе��ɸߵ��͵�˳���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009-2010ѧ��������ѧ�߶���ѧ����ĩ���Ի�ѧ ���ͣ������
(14��)A��B��C��DΪ���ֵ��ʣ�����ʱ��A��B�����壬C��D�ǹ��塣E��F��G��H��IΪ���ֻ����F����ˮ��EΪ�����Ҽ�����ˮ��Ϊ��ɫ��Һ��G��ˮ�û���ɫ��Һ����������ʼ䷴Ӧ��ת����ϵ��ͼ��ʾ

��1��д�����ֵ��ʵĻ�ѧʽ
A____ _____ B__________ C__ ____ D___ _____
��2��д��E��F��H��I�����ӷ���ʽ
��3��д��G+I��H+D+E�Ļ�ѧ����ʽ
��4��ij������B��Ư�ۡ�
��д����Ư�۵Ļ�ѧ����ʽ ��
��Ϊ�ⶨ�ù����Ƶõ�Ư������Ч�ɷֵĺ�����ij��С�����������ʵ�飺��ȡƯ��2.0g����ĥ���ܽ⣬���ó�250mL��Һ��ȡ��25.00mL���뵽��ƿ�У��ټ��������KI��Һ���������ᣨ��ʱ���������ӷ���ʽΪ�� �������á�����ȫ��Ӧ����0.1mol��L-1��Na2S2O3��Һ������Һ�ζ���Ӧ���ɵĵ⣬��֪��ӦʽΪ��2Na2S2O3+I2=Na2S4O6+2NaI������ȥNa2S2O3��Һ20.00mL�����Ư������Ч�ɷֵ���������Ϊ ��������С�������λ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com