
��17�֣��л���B������Է�������Ϊ46������̼����������Ϊ52.2%�������������Ϊ13.0%������Ϊ����A�IJ������Ժ���һ������ʯ�ͻ�����չˮƽ�� 
��1��B�ķ���ʽ�� ��A�Ľṹ��ʽ ��
��2��B��������Ʒ�Ӧ�ų���������B�ṹ�к��еĹ����ŵĵ���ʽΪ ��
��3����Ӧ����Cu�������������½��У��������� ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4����Ӧ�ٵķ�Ӧ������ ����Ӧ�۵Ļ�ѧ����ʽΪ ��
��5��B��������������Һ��Ӧ������D���ڼ��Ⱥ�Ũ���������£�B��D��Ӧ������һ������ζ������F����184 g B��120 g D��Ӧ������132g F���÷�Ӧ�Ļ�ѧ����ʽΪ ������B��ת����Ϊ ��
��1��B�ķ���ʽ��C2H6O��A�Ľṹ��ʽCH2=CH2����2�� ��
��
��3��Cu˿ʼ�ձ��ֺ��ȣ��������ɫ������ڡ���ڣ� ��
��
��4���ӳɷ�Ӧ��CH2=CH2��Br2��CH2BrCH2Br
��5��CH3COOH+C2H5OH CH3COOC2H5+H2O��37.5%��
CH3COOC2H5+H2O��37.5%��
���������������1��A����ϩCH2=CH2����ϩ��ˮ�����ӳɷ�Ӧ����B:�Ҵ�CH3CH2OH������ʽ��C2H6O���Ҵ��������õ�C����ȩCH3CHO����Ӧ����Cu�������������½��У���������Cu˿ʼ�ձ��ֺ��ȣ��������ɫ������ڡ���ڣ��÷�Ӧ�Ļ�ѧ����ʽΪ ����ȩ�������õ�D������CH3COOH����4����Ӧ�ٵķ�Ӧ�����Ǽӳɷ�Ӧ����ϩ���嵥�ʷ����ӳɷ�Ӧ����E��1,2���������飻��Ӧ�۵Ļ�ѧ����ʽΪCH2=CH2��Br2��CH2BrCH2Br�Ҵ���������Ũ���������·���������Ӧ����F����������CH3COOCH2CH3��ˮ���÷�Ӧ�ķ���ʽ��CH3COOH+C2H5OH
����ȩ�������õ�D������CH3COOH����4����Ӧ�ٵķ�Ӧ�����Ǽӳɷ�Ӧ����ϩ���嵥�ʷ����ӳɷ�Ӧ����E��1,2���������飻��Ӧ�۵Ļ�ѧ����ʽΪCH2=CH2��Br2��CH2BrCH2Br�Ҵ���������Ũ���������·���������Ӧ����F����������CH3COOCH2CH3��ˮ���÷�Ӧ�ķ���ʽ��CH3COOH+C2H5OH CH3COOC2H5+H2O��n(�Ҵ�)= 184 g��46g/mol=4mol��n(����)= 120 g��60g/mol=2mol��n(��������)= 132g��88g/mol=1.5mol,���ݷ�Ӧ����ʽ��֪��������1.5mol�����������ᷴӦ����1.5mol�Ҵ������Ҵ���ת����(1.5mol��4mol)��100%=37.5%��
CH3COOC2H5+H2O��n(�Ҵ�)= 184 g��46g/mol=4mol��n(����)= 120 g��60g/mol=2mol��n(��������)= 132g��88g/mol=1.5mol,���ݷ�Ӧ����ʽ��֪��������1.5mol�����������ᷴӦ����1.5mol�Ҵ������Ҵ���ת����(1.5mol��4mol)��100%=37.5%��
���㣺�����л���Ľṹ�����ʡ�ת������ѧ����ʽ�����ʵ�ת���ʵļ����֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��CH2=CH2��CH2BrCH2Br�ı仯����ͬһ��Ӧ���͵���( )
A��CH3CHO��C2H5OH B��C2H5OH��CH2=CH2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�л�����ˮ�����ķ�Ӧ��������
| A��ȡ����Ӧ | B���ӳɷ�Ӧ | C��ˮ�ⷴӦ | D����ȥ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѡ��5���л���ѧ��������15�֣�
��֪�л���A��B��C��D��E��F��G������ת����ϵ������C�IJ�������������һ�����ҵ�ʯ�ͻ�����չˮƽ��G�ķ���ʽΪC9H10O2���Իش������й����⡣
��1��G������Ϊ ��
��2��ָ�����з�Ӧ�ķ�Ӧ���ͣ�Aת��ΪB�� ��Cת��ΪD�� ��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
G������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ�� ��
��4����������������G��ͬ���칹����ĿΪ �֣�
�ٱ�������3��ȡ��������������ȡ������ͬ��
���ܹ������Ƶ�������Һ��Ӧ����������������
���к˴Ź������������ֲ�ͬ��ѧ����������������ʵĽṹ��ʽΪ �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��10�֣�һЩɱ������ʹ��ҩ�����ֲ������ӡ���Ƥ�����õ����綡��ӡ���Ƥ�ӣ���ṹ���£�
��ش�
��1������Ӻ͵�Ƥ�Ӷ�����ɱ�����ã���ԭ������Ǻ��������������� ��������ŵ����ƣ���
��2������Ӻ͵�Ƥ�Ӷ��ܷ����ķ�Ӧ����Ϊ������������ĸ��ţ���
| A���ӳɷ�Ӧ���� | B���Ӿ۷�Ӧ���������� | C��ȡ����Ӧ | D����ȥ��Ӧ |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��8�֣���1��������ͪ��һ���кɻ���ζ����ǿ�������õĻ�ѧ�Լ������Ľṹ��ʽ��ͼ��ʾ��������ͪ�����ܾ��еĻ�ѧ������ (����ĸ���)��
a���ӳɷ�Ӧ b��ȡ����Ӧ C����ȥ��Ӧd��ˮ�ⷴӦ e��������Ӧ f����ԭ��Ӧ
��2���ɱ�ϩ�����з�Ӧ���Ƶ�F��G���ָ߷��ӻ�������Ƕ��dz��õ����ϡ�
�پۺ���F�Ľṹ��ʽ�� ��
��c�����Ƶ�Cu(OH) ��������Һ����ת��ΪD�Ļ�ѧ����ʽ�ǣ� ��
��������Һ����ת��ΪD�Ļ�ѧ����ʽ�ǣ� ��
����һ�������£�������E����ȥ������ˮ�γ�һ����Ԫ��״������û�����Ľṹ��ʽ�ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��8�֣��ϳɸ߷��Ӳ�����;�㷺���������죬�书������ӽṹ�����еĹ�ϵ�������Ǽ��ָ߷��Ӳ��ϵĽṹ��ʽ��
��1��A����Ȼ����Ҫ�ɷ֣����ϻ���A�к��еĹ����ŵ�������____________��
��2��B�ĵ����DZ��Ӻ�___________�������ֵ����ڼ���£��ɵõ���״�߷��ӻ�����׳Ƶ�ľ�����ȹ������ϡ�
��3��C��NaOH��Һ��һ�������·�Ӧ�����ɸ���ˮ����֬������֬�Ľṹ��ʽ��___________________________________��
��4��D�Ǻϳ���ά��Ŀǰ������һ�ľ�����ά�������ڣ��������ֵ�����һ�������ºϳɣ��úϳɷ�Ӧ�����ֵ�����________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����֪��A��ʯ���ѽ�������Ҫ�ɷݣ�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ������AΪ��Ҫԭ�Ϻϳ�������������ϳ�·������ͼ��ʾ��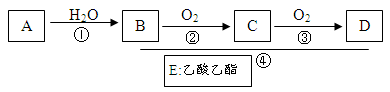
��1��A��һ�������¿��Ծۺ�����һ�ֳ������ϣ������ϵĽṹ��ʽΪ ��
��2����Ӧ�ܵĻ�ѧ����ʽΪ ��
��3����Ҫ��ش�
�١��ܵķ�Ӧ����Ϊ �� ���ڵķ�Ӧ����Ϊ ��
��4����ʵ�����л�õ�����������������B��D��Ϊ�ᴿE��������Լ��Լ�����������������ô����������̣��� �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(10��)2,3������ǿ�����ҩ����м��壬Ҳ���ڵ��ӻ�ѧƷ�����ϵ������У����ɱ�ϩΪԭ�Ϻϳɣ�
��֪����ૺ�����Ľṹ��ʽ�ֱ�Ϊ�� 
��±���� R��X RMgX
RMgX RCOMgX
RCOMgX RCOH��
RCOH��
�ش��������⣺
��1��ૡ�2,3����ૡ�������Ƿ�Ϊͬϵ���������������(��ǡ���)��
��2����ϵͳ����������������D��������������������
��3����Ӧ�۵�������________________��
��4��2,3����૿�ת��Ϊ����ૣ�����üĻ�ѧ�������� 2,3������Ƿ���ȫת��������
��5������ૻ����ɻ�����C4H10O2������Ũ����������Ƶã��÷�Ӧ�Ļ�ѧ����ʽΪ��____________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com