
�����14�֣�
���ж��ֺ����ᣬ�����ᣨH2SO3�������ᣨH2SO4���������ᣨH2SO4��SO3����������ᣨH2S2O3���ȵȣ�����������Ϊ��Ҫ���ڹ�ҵ���й㷺��Ӧ�á���ʵ���ң�Ũ�����dz��õĸ������
������м��㣺
(1)�����ᣨH2SO4��SO3������ˮ�����е�SO3��ת��Ϊ���ᡣ����445g����������ˮ���4.00L���ᣬ����������ʵ���Ũ��Ϊ________mol/��
(2)����Ũ������ˮ�����ɵ�H2SO4��H2O���㣬250g��������Ϊ98%�����������ն���gˮ��
(3)�������ǹ�ҵ�����������Ҫԭ�ϡ��������������յĻ�ѧ��Ӧ���£�
3FeS2��8O2��Fe3O4��6SO2 4FeS2��11 O2��2Fe2O3��8SO2
��48mol FeS2��ȫ��Ӧ��������2934.4L����״���������㷴Ӧ������Fe3O4��Fe2O3���ʵ���֮�ȡ�
(4)��������ȡ���ᣬ���ܳ��������Դ���ܱ�����������һ�ֺ��з�չǰ;���Ʊ�����ķ�����
�����������Ϊ0.84�Ļ�����壨H2S��H2O��N2���ڿ�������ȫȼ�գ�����������77%���������������SO2���������ˮ�����壩������֪������ɣ�N2�������0.79��O2�������0.21��
(1)1.25 (2)250��98%��98��16��40g
(3)2934.4��22.4��131mol
��Fe3O4 amol Fe2O3 bmol
3a+2b��48 8a+11��2b��131 a��4 b��18
n (Fe3O4):n(Fe2O3)��2:9
(4)������Ϊ1�����0.84���������ȫȼ������0.84������������0.84���ˮ������1.26����������������Ϊ1.26��0.21��1.77��10.62
���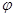 ��0.84�£�10.62��1.26+1.84����0.075
��0.84�£�10.62��1.26+1.84����0.075
�������������(1)445g����������ʵ�����445g��178g/mol��2.5mol����������������������2.5mol��2.5mol���������ֲ���2.5mol���ᣬ����Һ����������ʵ�����5.0mol��Ũ����5.0mol��4L��1.25mol/L��
(2)250g��������Ϊ98%�������������������250g��98%��245g�������ܼ�ˮ��5g����������ʵ�����245g��98g/mol��2.5mol�����Խ��ˮ�����ʵ�����2.5mol��������2.5mol��18g/mol��45g����˻���������ˮ��������45g��5g��40g��
(3)��Fe3O4�����ʵ�����amol��Fe2O3�����ʵ�����bmol
��Ӧ���������������ʵ�����2934.4L��22.4L/mol��131mol
����ݷ���ʽ��֪3a+2b��48��8a+11��2b��131
���a��4��b��18
��n (Fe3O4):n(Fe2O3)��2:9
(4)������Ϊ1����������Ȼ�����ȫȼ�յķ���ʽ2H2S��3O2��ȼ2SO2��2H2O ��֪��0.84���������ȫȼ������0.84������������0.84���ˮ������1.26�����������Ӧ�п�������77%������������Ϊ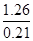 ��1.77��10.62������������������
��1.77��10.62������������������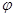 ��
��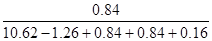 ��0.075
��0.075
���㣺�������仯���ﷴӦ���йػ�ѧ����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ���������β���к��е�������NOx��NO��NO2�Ļ������費��N2O4��������̬���������ཡ�������ϴ����в��
��1����ҵ�Ͽ��ð������շ�����NOx����Ӧԭ��Ϊ��
 ��ij��ѧ��ȤС��ģ��ô������̵�ʵ��װ�����£��г�װ������ȥ����
��ij��ѧ��ȤС��ģ��ô������̵�ʵ��װ�����£��г�װ������ȥ����
��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��װ��D�м�ʯ�ҵ������� ��
��2����ҵ��Ҳ����Na2CO3��Һ���շ�����NOx����֪��NO������Na2CO3��Һ��Ӧ��
NO+ NO2+Na2CO3=2NaNO2+ CO2��2NO2+Na2CO3= NaNO2+ NaNO3+CO2��
�ٵ�NOx��Na2CO3��Һ��ȫ����ʱ��x��ֵ�������� ������ĸ����
A��1.3 B��1.6 C��1.8
�ڽ�1 mol NOxͨ��Na2CO3��Һ�У�����ȫ����ʱ����Һ�����ɵ�NO��3��NO��2�������ӵ����ʵ�����x�仯��ϵ��ͼ��ʾ��
ͼ���߶�a��ʾ ������xֵ�仯�Ĺ�ϵ������������������Ϊ21.2%��Na2CO3��Һ���գ�����ҪNa2CO3��Һ���� g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ԫ������Ȼ���й㷺���ڣ��ش������йص����仯�����������⡣
��1����Ԫ���ǽϻ��õķǽ���Ԫ�أ���N2ȴ�����ã���ԭ���� ��������;�У�������N2���������ʵ���
�����ںϳɰ��� �ڽ�������ʱ�ı����� �۱���ʳƷ �ܺ�������������
���Ե���Ϊԭ��������
��2���£�N2H4�����������ȼ�ϣ������ķ�Ӧ�ǣ� N2O4(l) + 2N2H4(l)="==3" N2(g)+4H2O(g)
��֪N2H4�Ľṹ�ɿ���NH3��һ��H��-NH2���������N2H4����������ԭ���Ƿ���ͬһƽ���� ����ǡ��������÷�Ӧ����NA��N-H������ʱ���γɵĦм��� mol��
��3��ij���ʳ������������Ʊ�����泥�
��������������������ȥ��������ռ�����������İ������� %��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
�Ի�����Ϊԭ����������Ĺ�������ͼ���£�
��1����֪�� ��������Ϊ����£�SO3������H2O����ʱ�ų�������Ϊ ��������λ��Ч���֣���
��������Ϊ����£�SO3������H2O����ʱ�ų�������Ϊ ��������λ��Ч���֣���
��2������¯�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��3�����ݹ�������ͼ�ж�����˵����ȷ���ǣ�ѡ�������ĸ�� ��
a������¯�ų��Ŀ����ɹ�����
b���������������SO2��ת����
c��ʹ�ô��������SO2�ķ�Ӧ���ʺ�ת����
d��Ϊʹ��������ȼ�գ��轫�����
��4��������������SO3����ˮ��ϡ�����ԭ�� ��
��5���������ų���β�����ð�ˮ���գ�����Ũ���ᴦ�����õ��ϸ�Ũ�ȵ�SO2����Ρ�
��SO2�ȿ���Ϊ���������ԭ��ѭ�������ã�Ҳ�����ڹ�ҵ������������ճ�ʪ�����е�Br2��SO2����Br2�����ӷ���ʽ�� ��
��Ϊ�ⶨ������е�Ԫ�ص���������������ͬ��������ηֱ���뵽50.00mL��ͬŨ�ȵ�NaOH��Һ�У���ˮԡ����������ȫ���ݳ������¶�����β��ֽ⣩�������徭�������Ũ����������ȫ���ⶨŨ�������ӵ����������ֲⶨ������±���a>0���� 
���㣺������е�Ԫ�ص����������� %�����������Ϊ15.00g��Ũ�������ӵ�����Ϊ ����������������λС����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��19��2 g��Cu����������ϡ�����У�����Cu��ȫ��Ӧ��
��֪��3Cu + 8HNO3(ϡ) = 3Cu(NO3)2 +2NO��+ 4H2O��
��1���μӷ�Ӧ����������ʵ�����
��2������ԭ�������������
��3�����ɵ�NO�ڱ�״���µ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
̼���仯�����й㷺����;��
��1���ڵ绯ѧ�У�����̼���缫��
��������п�̸ɵ���У�̼���� ����
������̼�����������缫��ⱥ��ʳ��ˮ�����ռ�ʱ��̼���� ������Ӧ�����ӷ���ʽ ��
��2����ˮ����ͨ�����ȵ�̼�ɲ���ˮú����C(s)+H2O(g)  CO(g)+H2(g) ��H=+131.3kJ/mol,�ﵽƽ����������ʱ�������H2O��ƽ��ת���ʵĴ�ʩ�� ��
CO(g)+H2(g) ��H=+131.3kJ/mol,�ﵽƽ����������ʱ�������H2O��ƽ��ת���ʵĴ�ʩ�� ��
| A�������¶� | B������̼������ |
| C��������� | D����CO���ռ���ȥCO |
 CO2(g)+H2(g)�õ��������ݣ�
CO2(g)+H2(g)�õ��������ݣ�| �¶�/�� | ��ʼŨ��mol/L | ƽ��Ũ��mol/L | |
| CO(g) | H2O(g) | H2(g) | |
| 900 | 2.0 | 0.8 | 0.4 |
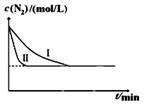
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijС��ͬѧ����ͼװ�ý���ʵ���о�(a��b��c��ʾֹˮ��)�������ۻ������䷽����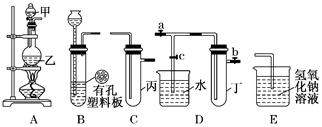
��װ��A��C��E�����ӣ���MnO2��Ũ������ȡ��������ش�
�٣���Ԫ�������ڱ��е�λ��Ϊ ��
�ڣ�A�з�Ӧ�����ӷ���ʽ��___ _��
�ۣ�E������������Һ������__________ ��
��C�м���������ˮ���Ƶ���ˮ����������ˮ�ֳ����ݽ���ʵ�飬�����������ͽ���Ϊ��
| ʵ����� | ʵ����� | ���� | ���� |
| �� | ����ˮ����Ʒ����Һ | Ʒ����Һ��ɫ | ������Ư���� |
| �� | ��ˮ�м���̼�����Ʒ�ĩ | ����ɫ���ݲ��� | ������ˮ��Ӧ�IJ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij����ѧϰС���ͬѧ�������ͼ��װ�ã�����֤SO2�������ԡ���ԭ�Ժ�Ư���ԡ�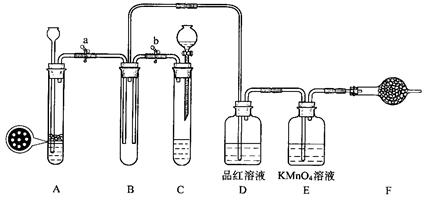
�ش��������⣺
33.�������װ��C�������ԵIJ����ǣ��رջ���b��Ȼ��________________________��
������________________��˵��װ��C���������á�
34.��Na2SO3�����������Һ��ȡSO2���壬Ӧѡ��__________��ѡ��A����C���������巢��װ�ã���ѡ����һװ�õ������� ��
35.С���ͬѧ��A��Cװ���е���һ����FeS�����ϡ������ȡH2S���壬��Ӧ�Ļ�ѧ����ʽΪ_________________ ��
36.SO2����ͨ��Dװ��ʱ������____________________ ��ͨ��Eװ��ʱ������
________________ ��SO2��H2S��Bװ���з�Ӧ��������______________________��
37.F��ʢ�м�ʯ�ң���������______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijʵ��С����̽��̼��Ũ���ᷴӦ���������ͬѧ�����ͼ1װ�ã���Ϊ���к���ɫ���������˵��̼��Ũ���ᷢ���˷�Ӧ��
��1����ͬѧ��Ϊ��ͬѧ�Ľ����Ǵ���ģ����������� (�û�ѧ����ʽ��ʾ)����������ΪӦ�ü��� (�ѧʽ)�IJ�����֤��̼��Ũ���ᷴӦ��Ϊ����ͬѧ����������ϵ�֪��0��ʱ����������ΪҺ�塱���Ӷ��Ľ���ʵ��װ����ͼ2��ʾ��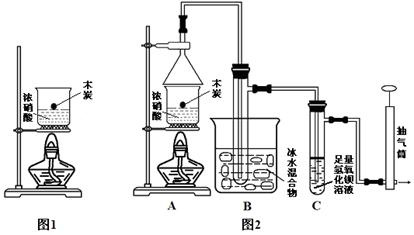
��2��Ϊ�˼��ٸ��ţ�����Aװ��������4�ֲ���������������������ǣ���д������ţ� ��
�ٽ�̿��Ũ����һͬ����װ���м��ȣ����ȼ���Ũ���ᣬȻ��̿Ͷ�����У�
���ȼ���̿���ټ�����Ũ��� ���ȼ���̿���ٽ�̿Ͷ����Ũ���ᡣ
��3������ƽ���ƶ�ԭ������Bװ�õ����� ��
��4��Cװ���г��ֵ������� �������C����Һ�л�����������Ԫ�أ�ֻ��NO3����ʽ���ڣ�д�����ɸ����ӵĻ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com