
(1)�״���һ����Ҫ�Ļ�����Ʒ�������ü״��������Ʊ���ȩ����ȩ����̬�״�ת����������ϵ��ͼ��ʾ��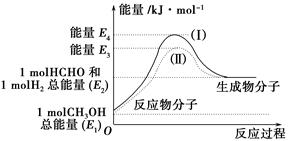
��Ӧ�����е�������ϵ
�ټ״�������ת��Ϊ��ȩ�ķ�Ӧ��________(����ȡ����ȡ�)��Ӧ��
�ڹ��̢�����̢�ķ�Ӧ���Ƿ���ͬ��____________ԭ����____________ ______________________________��
��д���״�������ת��Ϊ��ȩ���Ȼ�ѧ��Ӧ����ʽ______________ _____________________��
(2)��֪����CH3OH(g)��H2O(g)=CO2(g)��3H2(g)����H����49.0 kJ��mol��1
��CH3OH(g)��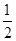 O2(g)=CO2(g)��2H2(g)����H����192.9 kJ��mol��1
O2(g)=CO2(g)��2H2(g)����H����192.9 kJ��mol��1
����˵����ȷ����________��
| A��CH3OHת���H2�Ĺ���һ��Ҫ�������� |
| B���ٷ�Ӧ�У���Ӧ�������������������������� |
C�����ݢ���֪��Ӧ��CH3OH(l)��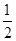 O2(g)=CO2(g)��2H2(g)�Ħ�H����192.9 kJ��mol��1 O2(g)=CO2(g)��2H2(g)�Ħ�H����192.9 kJ��mol��1 |
| D����Ӧ�ڵ������仯��ͼ��ʾ |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪��Ӧ��Fe(s)+CO2(g) FeO(s)+CO(g)����H=akJ��mol-1,ƽ�ⳣ��ΪK;
FeO(s)+CO(g)����H=akJ��mol-1,ƽ�ⳣ��ΪK;
��Ӧ��CO(g)+1/2O2(g) CO2(g)����H=bkJ��mol-1;
CO2(g)����H=bkJ��mol-1;
��Ӧ��Fe2O3(s)+3CO(g) 2Fe(s)+3CO2(g)����H=ckJ��mol-1������ڲ�ͬ�¶���,Kֵ����:
2Fe(s)+3CO2(g)����H=ckJ��mol-1������ڲ�ͬ�¶���,Kֵ����:
| �¶�/�� | 500 | 700 | 900 |
| K | 1.00 | 1.47 | 2.40 |
 2FeO(s)�Ħ�H=����������
2FeO(s)�Ħ�H=���������� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ĵ��зḻ����Ȼ����Դ������Ȼ��Ϊԭ�Ϻϳ����ص���Ҫ��������ͼ��ʾ(ͼ��ijЩת�����輰������δ�г�)��
����д���пհף�
(1)��֪0.5 mol������0.5 molˮ������t �桢p kPaʱ����ȫ��Ӧ����һ��
��̼������(�ϳ���)��������a kJ�������÷�Ӧ���Ȼ�ѧ����ʽ�ǣ�______________________��
(2)�ںϳɰ���ʵ�����������У�����ȡ�Ĵ�ʩ֮һ�ǣ������ɵİ��ӻ�������м�ʱ����������������������ĵ���������ѭ�����ã�ͬʱ���䵪���������������û�ѧ��Ӧ���ʺͻ�ѧƽ��Ĺ۵�˵����ȡ�ô�ʩ�����ɣ�
________________________________________________________________��
(3)������ϳɰ�����ת����Ϊ75%ʱ����5.60��107 L����Ϊԭ���ܹ��ϳ�________L������(����������ڱ�״���²ⶨ)
(4)��֪���صĽṹ��ʽΪ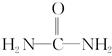 ����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��
����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��
��__________________�� ��_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���õ�����������һ�����������ɰ�����һ���淴Ӧ���ϳɰ�����һ����Ҫ�Ļ�����Ӧ������������Һ���Ͱ�ˮ��
���������գ�
��1����ͼ��ʾ�ϳɰ�ʱ����1mol������ʱ�������仯��E�ĵ�λΪkJ����д���ϳɰ����Ȼ�ѧ����ʽ____________________��
��������E1��E2��E3��ʾ������ͼ�е�ʵ�������߲�����ʲô��Ӧ���������˱仯��
��2����һ���¶��£�����4a mol H2��2amol N2����VL���ܱ������У�5���Ӻ���N2��ת����Ϊ50%����ö�ʱ����H2��ʾ�ķ�Ӧ����Ϊ__________Ħ��/(��?��)������ʱ�����������Ͷ��a mol H2��amol N2��2amol NH3���ж�ƽ���ƶ��ķ�����_____��������ƶ����������ƶ������ƶ�����
��3��Һ����ˮ���ƣ�Ҳ�ܵ��룺2NH3 NH4++ NH2����ij�¶�ʱ�������ӻ�K=2��l0-30�����¶��£��ٽ�����NH4Cl�������Һ���У�K____________2��10-30�����������������=�������ڽ�����������Ͷ��Һ���У���ȫ��Ӧ��������Һ�и�����Ũ�ȴ�С��ϵΪ��_______
NH4++ NH2����ij�¶�ʱ�������ӻ�K=2��l0-30�����¶��£��ٽ�����NH4Cl�������Һ���У�K____________2��10-30�����������������=�������ڽ�����������Ͷ��Һ���У���ȫ��Ӧ��������Һ�и�����Ũ�ȴ�С��ϵΪ��_______
��4�����������İ�ˮ������ʱ��Ҫϡ�͡���ˮϡ��0��1mol/Lϡ��ˮʱ����Һ������ˮ�������Ӷ����ٵ���
| A��c(NH4+)/c(NH3?H2O) | B��c(NH3?H2O)/c(OH-) |
| C��c(H+)/c(NH4+) | D��c(OH-)/c(H+) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
NOx������β���е���Ҫ��Ⱦ��֮һ��
(1)NOx���γ����꣬д��NO2ת��ΪHNO3�Ļ�ѧ����ʽ��__________________________��
(2)��������������ʱ������N2��O2��Ӧ���������仯ʾ��ͼ���£�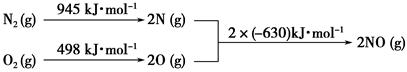
��д���÷�Ӧ���Ȼ�ѧ����ʽ��_______________________________��
�����¶����ߣ��÷�Ӧ��ѧƽ�ⳣ���ı仯������____��
(3)������β��ϵͳ��װ�ô�ת����������Ч����NOx���ŷš�
�ٵ�β���п�������ʱ��NOx�ڴ�ת�����б���ԭ��N2�ų���д��NO��CO��ԭ�Ļ�ѧ����ʽ��______________________________
�ڵ�β���п�������ʱ����ת�����еĽ�������������NOx�����Ρ�����������˳�����£�12MgO��20CaO��38SrO��56BaO��ԭ����___________________________________________��
Ԫ�صĽ���������ǿ�������������NOx��������������ǿ��
(4)ͨ��NOx�������ɼ��NOx�ĺ������乤��ԭ��ʾ��ͼ���£�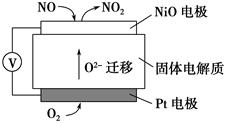
��Pt�缫�Ϸ�������________��Ӧ(���������ԭ��)
��д��NiO�缫�ĵ缫��Ӧʽ��______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
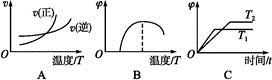
����Cu2O���ھ��������Ĵ����ܶ��ܵ���ע���±�Ϊ��ȡCu2O�����ַ�����
| ������ | ��̿���ڸ��������»�ԭCuO |
| ������ | ��ⷨ����ӦΪ2Cu + H2O  Cu2O + H2���� Cu2O + H2���� |
| ������ | ���£�N2H4����ԭ����Cu(OH)2 |
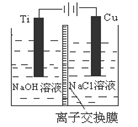
 ��H >0
��H >0| ��� | �¶� | 0 | 10 | 20 | 30 | 40 | 50 |
| �� | T1 | 0.050 | 0.0492 | 0.0486 | 0.0482 | 0.0480 | 0.0480 |
| �� | T1 | 0.050 | 0.0488 | 0.0484 | 0.0480 | 0.0480 | 0.0480 |
| �� | T2 | 0.10 | 0.094 | 0.090 | 0.090 | 0.090 | 0.090 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ԫ�صĻ���������࣬����Ҳ������ͬ��
��1��NO2�н�ǿ�������ԣ��ܽ�SO2��������SO3����������ԭΪNO����֪��������Ӧ�����������仯��ͼ��ʾ��
��NO2����SO2���Ȼ�ѧ����ʽΪ_________________________________��
��2����2L�ܱ������з���1mol��������һ���¶Ƚ������·�Ӧ��
2NH3(g) N2��g��+3H2��g������Ӧʱ��(t)��������������ѹǿ(p)�����ݼ��±�
N2��g��+3H2��g������Ӧʱ��(t)��������������ѹǿ(p)�����ݼ��±�
| ʱ��t/min | 0 | 1 | 2 | 3 | 4 | 5 |
| ��ѹǿp 100 kPa | 5 | 5.6 | 6.4 | 6.8 | 7 | 7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Ϊһ������Դ�ڻ�ѧ����Ӧ�ù㷺����ش��������⡣
��1����¯ұ�������У������ڴ���Ӧ���в���ˮú����CO��H2����ԭ���������йط�ӦΪ��CH4��g����CO2��g��=2CO��g����2H2��g������H��260 kJ��mol��1
��֪��2CO��g����O2��g��=2CO2��g����H����566 kJ��mol��1��
��CH4��O2��Ӧ����CO��H2���Ȼ�ѧ����ʽΪ____________________________________��
��2������ͼ��ʾ��װ�â�Ϊ����ȼ�ϵ�أ��������ҺΪKOH��Һ����ͨ��װ�â�ʵ�������϶�ͭ��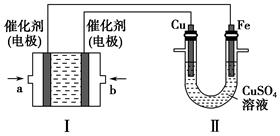
��a��Ӧͨ��________���CH4����O2������b���缫�Ϸ����ĵ缫��Ӧʽ��_________________________________________________________________��
�ڵ�ƽ�����װ�â�����Һ��pH________����д�������С�����䡱����ͬ����װ�â���Cu2�������ʵ���Ũ��________��
�۵�ƽ�����װ�â���Һ�е������ӳ���OH���������________������ˮ�⣩��
���ڴ˹���������ȫ��Ӧ��װ�â������������仯12.8 g����װ�â������������ļ���________L����״���£���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���̼�Ȼ�ԭ���Ȼ�����ʵ�����������Ʊ�������,����ط�Ӧ���Ȼ�ѧ����ʽ����:
Al2O3(s)+AlCl3(g)+3C(s) 3AlCl(g)+3CO(g)����H="a" kJ��mol-1
3AlCl(g)+3CO(g)����H="a" kJ��mol-1
3AlCl(g) 2Al(l)+AlCl3(g)����H="b" kJ��mol-1
2Al(l)+AlCl3(g)����H="b" kJ��mol-1
��ӦAl2O3(s) +3C(s) 2Al(l)+3CO(g)�Ħ�H=�������� kJ��mol-1(�ú�a��b�Ĵ���ʽ��ʾ)��
2Al(l)+3CO(g)�Ħ�H=�������� kJ��mol-1(�ú�a��b�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com