
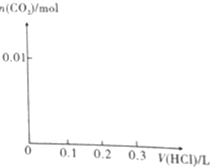
 ��1����0.4gNaOH��1.06gNa2CO3��ϲ������Һ������Һ�еμ�0.1mol•L-1ϡ���ᣮ����ͼ��ʾ����ϵ�л�������ȷ��ʾ������������������CO2�����ʵ����Ĺ�ϵͼ��
��1����0.4gNaOH��1.06gNa2CO3��ϲ������Һ������Һ�еμ�0.1mol•L-1ϡ���ᣮ����ͼ��ʾ����ϵ�л�������ȷ��ʾ������������������CO2�����ʵ����Ĺ�ϵͼ������ ��1����0.4gNaOH�����ʵ���Ϊ��0.01mol��1.06gNa2CO3�����ʵ���Ϊ��0.01mol������Һ�еμ�0.1mol•L-1ϡ���ᣬ�������������������Ʒ�Ӧ�����Ȼ�����ˮ��������������Ϊ��$\frac{0.01mol}{0.1mol/L}$=0.1L�������μ�ʱ�������Na2CO3��ʼ��Ӧ�����ȷ���HCl+Na2CO3=NaHCO3+NaCl�����ų����壬����������ΪΪ0.1 Lʱ̼����ȫ��ת��Ϊ̼�����ƣ������μ�ʱ��������Ӧ��NaHCO3+HCl=NaCl+H2O+CO2������ʱ�����������0.1L���ɴ˷�����ͼ��
��2�����ȷ�����Ӧ��NaOH+NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+H2O����̼�����ƹ�������������Ӧ��NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+CO2��+H2O����NaOH��NaHCO3ǡ�ð�1��1��Ӧ�������������Ϊˮ�����������ݷ���ʽ��֪������ˮ������=18.4g��$\frac{18}{124}$=2.67g��ʵ�ʹ�����������=18.4g-16.6g=1.8g��2.67g�������������ƹ�����̼��������ȫ��Ӧ�����ݷ���ʽ����NaHCO3����������������NaOH���������ټ����������Ƶ�����������
��� �⣺��1����0.4gNaOH�����ʵ���Ϊ��0.01mol��1.06gNa2CO3�����ʵ���Ϊ��0.01mol������Һ�еμ�0.1mol•L-1ϡ���ᣬ�������������������Ʒ�Ӧ�����Ȼ�����ˮ��������������Ϊ��$\frac{0.01mol}{0.1mol/L}$=0.1L�������μ�ʱ�������Na2CO3��ʼ��Ӧ�����ȷ���HCl+Na2CO3=NaHCO3+NaCl�����ų����壬����������ΪΪ0.1 Lʱ̼����ȫ��ת��Ϊ̼�����ƣ������μ�ʱ��������Ӧ��NaHCO3+HCl=NaCl+H2O+CO2������ʱ�����������0.1L��ͼ��Ϊ��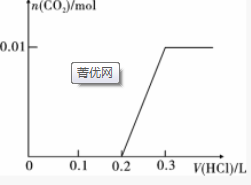 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2�����ȷ�����Ӧ��NaOH+NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+H2O����̼�����ƹ�������������Ӧ��NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+CO2��+H2O��
��NaOH��NaHCO3ǡ�ð�1��1��Ӧ�������������Ϊˮ��������������ˮ������Ϊm����
NaOH+NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+H2O
40 84 18
18.4 m
m=18.4g��$\frac{18}{124}$=2.67g��ʵ�ʹ�����������=18.4g-16.6g=1.8g��2.67g�������������ƹ�����̼��������ȫ��Ӧ��
��NaHCO3������Ϊa����
NaOH+NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+H2O
84 18
n 1.8g
��a=$\frac{1.8g��84}{18}$=8.4g
�������NaOH��������18.4g-8.4g=10g���������NaOH��������=$\frac{10g}{18.4g}$��100%=54.35%��
�𣺻������NaOH����������Ϊ54.35%��
���� ���⿼��������㡢��ѧ����ʽ���㣬���ü��跨�жϹ��������ǽ���ؼ����Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2H2��+O2����2H2+O2$\frac{\underline{\;��ȼ\;}}{\;}$2H2O | |
| B�� | NH3+CO2+H2O�TNH4HCO3��NH4HCO3$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O��+CO2�� | |
| C�� | H2+I2$\frac{\underline{\;\;��\;\;}}{\;}$2HI��2HI$\frac{\underline{\;\;��\;\;}}{\;}$H2��+I2�� | |
| D�� | 2Ag+Br2$\frac{\underline{\;CuO\;}}{\;}$2AgBr��2AgBr$\frac{\underline{\;��\;}}{\;}$2Ag+Br2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ǵڢ���Ԫ�� | |
| B�� | ���ǵ�������Ԫ�� | |
| C�� | �����ڹ���Ԫ�� | |
| D�� | ����ij��ͬλ�غ������������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Fe3O4��������ϡHNO3��Fe3O4+8H+=Fe2++2Fe3++4H2O | |
| B�� | NH4HCO3��Һ������Ba��OH��2��Һ��ϣ�HCO3-+Ba2++OH-=BaCO3��+H2O | |
| C�� | ���Ƶ�ˮ�ⷴӦ��S2-+H3O+HS-+H2O | |
| D�� | ��0.2 mol•L-1��NH4Al��SO4��2��Һ��0.3 mol•L-1��Ba��OH��2��Һ�������ϣ�2Al3++3SO42-+3Ba2++6OH-=2Al��OH��3��+3BaSO4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������� | B�� | ��������� | C�� | ������Ӳ��� | D�� | ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��A��͢�A��Ԫ���γɵ�ԭ�Ӹ�����Ϊ1��1����������Ϊ38�Ļ�����Ǻ����ۼ������ӻ����� | |
| B�� | ��SiO2�����У�1����ԭ�Ӻ�2����ԭ���γ�2�����ۼ� | |
| C�� | HI����Է���������HF������HI�ķе��HF�� | |
| D�� | H��D��16O��18O��Ϊͬλ�أ�H216O��D216O��H218O��D216O��Ϊͬ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
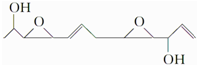 ���й��ڸû������˵����ȷ���ǣ�������
���й��ڸû������˵����ȷ���ǣ�������| A�� | ����ʽΪC12H19O4 | B�� | ���Ҵ���ͬϵ�� | ||
| C�� | �ɷ���������Ӧ | D�� | ����ͬһƽ���ԭ�������5�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com