
(14Ζ÷)ΝρΥαΙΛ“Β÷–2SO2(g)ΘΪO2(g)¥ΏΜ·ΦΝΓς2SO3(g)ΘΜΠΛH<0(Ζ≈»»Ζ¥”Π)”–ΙΊ Β―ι ΐΨί»γœ¬ΘΚ
  ―Ι«Ω ―Ι«ΩSO2ΒΡ ΉΣΜ·¬ Έ¬Ε» | 1ΓΝ105 Pa | 5ΓΝ105 Pa | 10ΓΝ105 Pa | 50ΓΝ105 Pa | 100ΓΝ105 Pa |
| 450 Γφ | 97.5% | 98.9% | 99.2% | 99.6% | 99.7% |
| 550 Γφ | 85.6% | 92.9% | 94.9% | 97.7% | 98.3% |
 ΒΡΩ’Τχ «ΈΣΝΥ________ΓΘ
ΒΡΩ’Τχ «ΈΣΝΥ________ΓΘΘ®14Ζ÷Θ©ΓΓ(1) ΙΤΫΚβ’ΐœρ“ΤΕ·Θ§ΧαΗΏSO2ΒΡΉΣΜ·¬
(2)ΗΏΈ¬ ΙΖ¥”ΠΥΌ¬ Φ”ΩλΘ§ΥθΕΧΝΥ¥οΒΫΤΫΚβΥυ–ηΒΡ ±ΦδΘ§ΒΪ «Ε‘SO2ΒΡΉΣΜ·≤ΜάϊΓΓΓΘ
¥ΏΜ·ΦΝ‘ΎΗΟΈ¬Ε»œ¬Μν–‘Ήν«ΩΘ§¥ΏΜ·–ßΙϊΉνΦ―ΓΘ
(3)Φ”ΩλΖ¥”ΠΥΌ¬ Θ§«“ ΙΤΫΚβ’ΐœρ“ΤΕ·Θ§”–άϊ”ΎSO2ΉΣΜ·ΚΆSO3ΒΡ…ζ≥…ΓΘΓΓ
≥Θ―Ι ±SO2ΒΡΉΣΜ·¬ “―Ψ≠ΚήΗΏΘ§Έό≤…”ΟΗΏ―ΙΒΡ±Ί“ΣΘ§Ωω«“Θ§≤…”ΟΗΏ―ΙΜΙ ήΕ·ΝΠΓΔ…η±Η Β»ΧθΦΰΒΡœό÷ΤΘ§ΧαΗΏΝΥ≥…±ΨΓΘ
Β»ΧθΦΰΒΡœό÷ΤΘ§ΧαΗΏΝΥ≥…±ΨΓΘ
(4)”ΟΥ°Έϋ ’SO3“Ή–Έ≥…ΥαΈμΘ§Έϋ ’–ßΙϊ≤νΘ§Εχ”Ο≈®H2SO4Έϋ ’‘ρ≤Μ“Ή–Έ≥…ΥαΈμΘ§Έϋ ’–ßΙϊΚΟΓΘΓΓ
Ζά÷ΙΩ’ΤχΈέ»ΨΓΘ
ΫβΈω



| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
(14Ζ÷)ΝρΥαΙΛ“Β÷–2SO2(g)ΘΪO2(g)¥ΏΜ·ΦΝΓς2SO3(g)ΘΜΠΛH<0(Ζ≈»»Ζ¥”Π)”–ΙΊ Β―ι ΐΨί»γœ¬ΘΚ
|
SO2ΒΡ ΉΣΜ·¬ Έ¬Ε» | 1ΓΝ105 Pa | 5ΓΝ105 Pa | 10ΓΝ105 Pa | 50ΓΝ105 Pa | 100ΓΝ105 Pa |
| 450 Γφ | 97.5% | 98.9% | 99.2% | 99.6% | 99.7% |
| 550 Γφ | 85.6% | 92.9% | 94.9% | 97.7% | 98.3% |
(1)‘Ύ…ζ≤ζ÷–≥Θ”ΟΙΐΝΩΒΡΩ’Τχ «ΈΣΝΥ________ΓΘ
(2)ΗΏΈ¬Ε‘ΗΟΖ¥”Π”–ΚΈ”ΑœλΘΩ________Θ§ ΒΦ …ζ≤ζ÷–≤…”Ο400ΓΪ500 ΓφΒΡΈ¬Ε»≥ΐΝΥΩΦ¬«ΥΌ¬ “ρΥΊΆβΘ§ΜΙΩΦ¬«ΒΫ________ΓΘ
(3)‘ω¥σ―Ι«ΩΕ‘…œ ωΖ¥”Π”–ΚΈ”ΑœλΘΩ____Θ§ΒΪΙΛ“Β…œ”÷≥Θ≤…”Ο≥Θ―ΙΫχ––Ζ¥”ΠΘ§Τδ‘≠“ρ «______________ΓΘ
(4)≥Θ”Ο≈®H2SO4Εχ≤Μ”ΟΥ°Έϋ ’SO3 «”…”Ύ___ ___Θ§Έ≤Τχ÷–SO2±Ί–κΜΊ ’Θ§÷ς“Σ «ΈΣΝΥ________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2012Ϋλ…ΫΕΪ ΓΈΔ…Ϋ“Μ÷–ΗΏ»ΐ10‘¬‘¬ΩΦΜ·―ß ‘Ψμ Χβ–ΆΘΚ Β―ιΧβ
(14Ζ÷)ΝρΥαΙΛ“ΒΈ≤Τχ÷–Εΰ―θΜ·ΝρΒΡΚ§ΝΩ≥§Ιΐ0.05%(ΧεΜΐΖ÷ ΐ) ±–ηΨ≠¥ΠάμΚσ≤≈Ρή≈≈Ζ≈ΓΘΡ≥–ΘΜ·―ß–Υ»Λ–ΓΉι”ϊ≤βΕ®Ρ≥ΝρΥαΙΛ≥ß≈≈Ζ≈Έ≤Τχ÷–Εΰ―θΜ·ΝρΒΡΚ§ΝΩΘ§Ζ÷±π≤…”Ο“‘œ¬ΖΫΑΗΘΚ
[ΦΉΖΫΑΗ]ΘΚ»γΆΦΥυ ΨΘ§ΆΦ÷–ΤχΧεΝςΝΩΦΤB”Ο”ΎΉΦ»Ζ≤βΝΩΆ®ΙΐΒΡΈ≤ΤχΧεΜΐΓΘΫΪΈ≤ΤχΆ®»κ“ΜΕ®ΧεΜΐ“―÷Σ≈®Ε»ΒΡΒβΥ°÷–≤βΕ®SO2ΒΡΚ§ΝΩΓΘΒ±œ¥ΤχΤΩC÷–»ή“ΚάΕ…Ϊœϊ ß ±Θ§ΝΔΦ¥ΙΊ±’Μν»ϊAΓΘ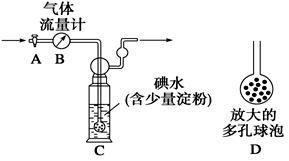
(1)œ¥ΤχΤΩC÷–ΒΦΙήΡ©ΕΥΝ§Ϋ”“ΜΗωΕύΩΉ«ρ≈ίDΘ§Ω…“‘ΧαΗΏ Β―ιΒΡΉΦ»ΖΕ»Θ§Τδάμ”… «_______________________________________ΓΘ
(2)œ¥ΤχΤΩC÷–»ή“ΚάΕ…Ϊœϊ ßΚσΘ§ΟΜ”–ΦΑ ±ΙΊ±’Μν»ϊAΘ§≤βΒΟΒΡSO2Κ§ΝΩ____________(ΧνΓΑΤΪΗΏΓ±ΓΔΓΑΤΪΒΆΓ±ΜρΓΑΈό”ΑœλΓ±)ΓΘ
[““ΖΫΑΗ]ΘΚ Β―ι≤Ϋ÷η»γœ¬ΟφΝς≥ΧΆΦΥυ ΨΘΚ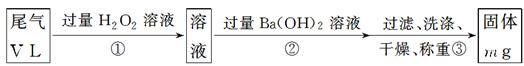
(3)≤Ϋ÷ηΔΌ÷–ΙΐΝΩH2O2ΒΡΉς”Ο «
(4)–¥≥ω≤Ϋ÷ηΔΎ÷–Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ_______________________________________
(5)≤Ϋ÷ηΔΎ÷–Ba(OH)2 «ΖώΉψΝΩΒΡ≈–ΕœΖΫΖ® «________________________________
(6)Ά®ΙΐΒΡΈ≤ΤχΧεΜΐΈΣV L(“―ΜΜΥψ≥…±ξΉΦΉ¥Ωω) ±Θ§ΗΟΈ≤Τχ÷–Εΰ―θΜ·ΝρΒΡΚ§ΝΩ(ΧεΜΐΖ÷ ΐ)ΈΣ__________________________(”ΟΚ§”–VΓΔmΒΡ¥ζ ΐ Ϋ±μ Ψ)ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2012-2013―ßΡξΚΰΡœ ΓΗΏ»ΐΒΎ»ΐ¥ΈΫΧ”ΐ÷ ΝΩΦλ≤βΜ·―ß ‘ΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚΧνΩ’Χβ
(14Ζ÷)―ΈΥαΓΔΝρΥαΚΆœθΥαΕΦ «÷Ί“ΣΒΡΜ·ΙΛ‘≠ΝœΘ§“≤ «Μ·―ß Β―ι “άο±Ί±ΗΒΡ÷Ί“Σ ‘ΦΝΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©≥ΘΈ¬œ¬Θ§Ω…”ΟΧζΓΔ¬Ν÷ΤΒΡ»ίΤς ΔΖ≈≈®ΝρΥαΘ§ΥΒΟς≈®ΝρΥαΨΏ”– –‘ΓΘ”Ο≤ΘΝßΑτ’Κ»Γ≈®ΝρΥαΒΈ‘Ύ÷Ϋ…œΘ§÷Ϋ÷πΫΞ±δΚΎΘ§ΥΒΟς≈®ΝρΥαΨΏ”– –‘ΓΘ
Θ®2Θ©œθΥαΆ≠ «÷Τ±ΗCu-Zn-AlœΒ¥ΏΜ·ΦΝΒΡ÷Ί“Σ‘≠ΝœΘ§ΙΛ“Β…œ”Οœ¥ΨΜΒΡΖœΆ≠–ΦΉς‘≠Νœά¥÷Τ±ΗœθΥαΆ≠ΓΘœ¬Ν–÷Τ±ΗΖΫΖ®ΖϊΚœΓΑ¬Χ…ΪΜ·―ßΓ±ΥΦœκΒΡ « Θ®Χν–ρΚ≈Θ©ΓΘ
ΔΌ Cu + HNO3Θ®≈®Θ©Γζ Cu(NO3)2 ΔΎ Cu + HNO3Θ®œΓΘ©Γζ Cu(NO3)2
Δέ Cu CuO
CuO Cu(NO3)2
Cu(NO3)2
Θ®3Θ©ΔΌ‘Ύ100mL 18molΓΛL-1ΒΡ≈®ΝρΥα÷–Φ”»κΙΐΝΩΒΡΆ≠Τ§Θ§Φ”»» Ι÷°≥δΖ÷Ζ¥”ΠΘ§≤βΒΟ≤ζ…ζΒΡΤχΧε‘Ύ±ξΉΦΉ¥Ωωœ¬ΒΡΧεΜΐΩ…Ρή « ΓΘ
AΘ°40.32L BΘ°30.24L CΘ°20.16L DΘ°13.44L
ΔΎ»τ Ι…œ ωΖ¥”ΠΔΌ÷– Θ”ύΒΡΆ≠Τ§ΦΧ–χ»ήΫβΘ§Ω…œρΤδ÷–Φ”»κœθΥαΡΤΘ§–¥≥ωΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ ΓΘ
Θ®4Θ©»τΫΪ12.8gΆ≠Ηζ“ΜΕ®÷ ΝΩΒΡ≈®HNO3Ζ¥”Π,Ά≠œϊΚΡΆξ ±,Ι≤≤ζ…ζΤχΧε5.6L(±ξΉΦΉ¥Ωω),‘ρΥυΚΡHNO3ΒΡΈο÷ ΒΡΝΩ mol
(5)Ρ≥Ά§―ßœρΫΰ≈ίΆ≠Τ§ΒΡœΓ―ΈΥα÷–Φ”»κH2O2ΚσΘ§Ά≠Τ§»ήΫβΘ§≤Δ«“ΗΟΖ¥”ΠΒΡ≤ζΈο÷Μ”–¬»Μ·Ά≠ΚΆΥ°ΓΘΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2013Ϋλ÷Ί«λ –ΗΏΕΰ…œ―ßΤΎΤΎ÷–ΩΦ ‘Μ·―ß ‘Ψμ Χβ–ΆΘΚΧνΩ’Χβ
(14Ζ÷)ΝρΥαΙΛ“Β÷–2SO2(g)ΘΪO2(g)¥ΏΜ·ΦΝΓς2SO3(g)ΘΜΠΛH<0(Ζ≈»»Ζ¥”Π)”–ΙΊ Β―ι ΐΨί»γœ¬ΘΚ
|
SO2ΒΡ ΉΣΜ·¬ Έ¬Ε» |
1ΓΝ105 Pa |
5ΓΝ105 Pa |
10ΓΝ105 Pa |
50ΓΝ105 Pa |
100ΓΝ105 Pa |
|
450 Γφ |
97.5% |
98.9% |
99.2% |
99.6% |
99.7% |
|
550 Γφ |
85.6% |
92.9% |
94.9% |
97.7% |
98.3% |
(1)‘Ύ…ζ≤ζ÷–≥Θ”ΟΙΐΝΩΒΡΩ’Τχ «ΈΣΝΥ________ΓΘ
(2)ΗΏΈ¬Ε‘ΗΟΖ¥”Π”–ΚΈ”ΑœλΘΩ________Θ§ ΒΦ …ζ≤ζ÷–≤…”Ο400ΓΪ500 ΓφΒΡΈ¬Ε»≥ΐΝΥΩΦ¬«ΥΌ¬ “ρΥΊΆβΘ§ΜΙΩΦ¬«ΒΫ________ΓΘ
(3)‘ω¥σ―Ι«ΩΕ‘…œ ωΖ¥”Π”–ΚΈ”ΑœλΘΩ____Θ§ΒΪΙΛ“Β…œ”÷≥Θ≤…”Ο≥Θ―ΙΫχ––Ζ¥”ΠΘ§Τδ‘≠“ρ «______________ΓΘ
(4)≥Θ”Ο≈®H2SO4Εχ≤Μ”ΟΥ°Έϋ ’SO3 «”…”Ύ___ ___Θ§Έ≤Τχ÷–SO2±Ί–κΜΊ ’Θ§÷ς“Σ «ΈΣΝΥ________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΙζΦ ―ß–Θ”≈―Γ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com