
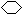 ��1�֣� ��(CH3)3CCH=CH2��CH2=C(CH3)CH(CH3)2��(CH3)2C=C(CH3)2��ÿ��2�֣���
��1�֣� ��(CH3)3CCH=CH2��CH2=C(CH3)CH(CH3)2��(CH3)2C=C(CH3)2��ÿ��2�֣���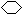 ��
��

 ��ʱѵ���������������ϵ�д�
��ʱѵ���������������ϵ�д� �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д� ����С����ҵ��ϵ�д�
����С����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������������̼ԭ����һ��ֱ���� |
| B����5��̼ԭ�ӵ��л�������������γ�4��C��C���� |
| C������������ͬ���칹�� |
| D�����ȼ���������ͬ���칹�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CnH2n+2O�Ĵ� | B��CnH2nO��ȩ | C��CnH2nO2������ | D��CnH2n+2���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
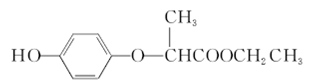
| A����1 mol HPE����Һ�����Ժ�2 mol NaOH��Ӧ |
| B��HPE�ܸ�Ũ��ˮ��Ӧ������ɫ���� |
| C���û������ں˴Ź�����������ʾ��5�������� |
| D��������Ϊ�����廯�������ʽΪC11H12O4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢ� | B���٢� | C���ڢۢ� | D���ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��MVNO/22.4w | B��wVNO/22.4M | C��wVNO/M | D��2VNO/Mw |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
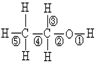
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ����ѧʽΪC8H10O���ж���ͬ���칹�壬�������ڷ��㴼��ͬ���칹��һ���У� ����
����ѧʽΪC8H10O���ж���ͬ���칹�壬�������ڷ��㴼��ͬ���칹��һ���У� ���� | A��6�֡��� | B��5�֡��� | C��4�֡��� | D��3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
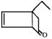
A�� | B��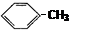 |
C��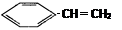 | D��CH3��CH�TCH��CH3 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com