
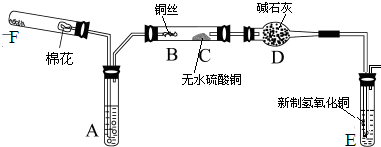
��10�֣���ͼ��ʾΪij��ѧ��ȤС����Ƶ��Ҵ������������������ʵ��װ��(ͼ�м�������������̨�����еȾ�δ����)��
ͼ�У�AΪ��ˮ�Ҵ�(�е�Ϊ78��)��BΪ�Ƴ�����״��ϸͭ˿����˿��CΪ��ˮCuSO4��ĩ��DΪ��ʯ�ң�FΪ���Ƶļ���Cu(OH)2����Һ��
(1)������װ���У�ʵ��ʱ��Ҫ���ȵ�����Ϊ(��������ij��λ�Ĵ���) ��
(2)ΪʹA���Ҵ�ƽ���������Ҵ�����,�����õķ�����_____________��
D��ʹ�ü�ʯ�ҵ������� ��
(3)�����Ҵ���������ʱF�е�ʵ�������� ��
(4)E����һ�ִ�����䷴Ӧ����ʽΪ ��
(5)д���Ҵ������������Ļ�ѧ����ʽ ��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� |
| ���� |
| Cu |
| ���� |
| Cu |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��2011ѧ��ɽ��ʡΫ�������ظ߶���ѧ����ĩ������ѧ�Ծ� ���ͣ�ʵ����
��12�֣���ͼ��ʾΪij��ѧ��ȤС����Ƶ��Ҵ�������ʵ��װ��(ͼ�м�������������̨�����еȾ�δ����)��
ͼ�У�AΪ��ˮ�Ҵ�(�е�Ϊ78��)��BΪ�Ƴ�����״��ϸͭ˿��CΪ��ˮCuSO4��ĩ��DΪ��ʯ�ң�FΪ���Ƶļ���Cu(OH)2����Һ��
(1) E����һ�ִ�����䷢����Ӧ�Ļ�ѧ����ʽΪ___ _____��
(2)ΪʹA���Ҵ�ƽ���������Ҵ������������õķ�����_______ ______��
D��ʹ�ü�ʯ�ҵ�������__ _____��
(3)��֤���Ҵ���Ӧ������ʵ��������
��
(4)��ʵ������������E���������������䣬����C�������Ա仯����F��������(3)��ͬ���ƶ�B��������Ӧ�Ļ�ѧ����ʽ____ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
��12�֣���ͼ��ʾΪij��ѧ��ȤС����Ƶ��Ҵ�������ʵ��װ��(ͼ�м�������������̨�����еȾ�δ����)��
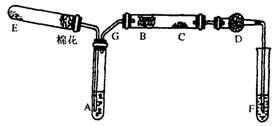
ͼ�У�AΪ��ˮ�Ҵ�(�е�Ϊ78��)��BΪ�Ƴ�����״��ϸͭ˿��CΪ��ˮCuSO4��ĩ��DΪ��ʯ�ң�FΪ���Ƶļ���Cu(OH)2����Һ��
(1) E����һ�ִ�����䷢����Ӧ�Ļ�ѧ����ʽΪ___ _____��
(2)ΪʹA���Ҵ�ƽ���������Ҵ������������õķ�����_______ ______��
D��ʹ�ü�ʯ�ҵ�������__ _____��
(3)��֤���Ҵ���Ӧ������ʵ��������
��
(4)��ʵ������������E���������������䣬����C�������Ա仯����F��������(3)��ͬ���ƶ�B��������Ӧ�Ļ�ѧ����ʽ____ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��09-10ѧ��ӱ�ʡ��һ�꼶��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
��10�֣���ͼ��ʾΪij��ѧ��ȤС����Ƶ��Ҵ������������������ʵ��װ��(ͼ�м�������������̨�����еȾ�δ����)��

ͼ�У�AΪ��ˮ�Ҵ�(�е�Ϊ78��)��BΪ�Ƴ�����״��ϸͭ˿����˿��CΪ��ˮCuSO4��ĩ��DΪ��ʯ�ң�FΪ���Ƶļ���Cu(OH)2����Һ��
(1)������װ���У�ʵ��ʱ��Ҫ���ȵ�����Ϊ(��������ij��λ�Ĵ���) ��
(2)ΪʹA���Ҵ�ƽ���������Ҵ�����,�����õķ�����_____________��
D��ʹ�ü�ʯ�ҵ������� ��
(3)�����Ҵ���������ʱF�е�ʵ�������� ��
(4) E����һ�ִ�����䷴Ӧ����ʽΪ ��
(5)д���Ҵ������������Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com