��2013?������һģ����������;�㷺�Ļ���ԭ�ϣ�������ˮ������ˮ�����������ʹ����ȣ�
��1����ҵ������ͭ�ķ����࣮ܶ
�ٷ���һ����Ũ�����ͭ��ȡ����ͭ���÷�Ӧ�Ļ�ѧ����ʽ��
Cu+2H
2SO
4��Ũ��
CuSO
4+SO
2��+2H
2O
Cu+2H
2SO
4��Ũ��
CuSO
4+SO
2��+2H
2O
���˷������ȱ����
��������Ⱦ������
��������Ⱦ������
��
�ڷ���������ϡ���ᡢͭ����������ȡ����ͭ����������Ҫ������ͼ��ʾ��
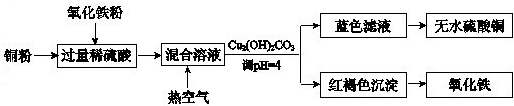
ϡ���ᡢͭ����������Ӧ�Ļ�ѧ����ʽ��
Fe2O3+6H+�T2Fe3++3H2O��2Fe3++Cu�T2Fe2++Cu2+��
Fe2O3+6H+�T2Fe3++3H2O��2Fe3++Cu�T2Fe2++Cu2+��
��������Һ��ͨ���ȿ����ķ�Ӧ�����ӷ���ʽ��
4Fe2++4H++O2�T4Fe2++2H2O
4Fe2++4H++O2�T4Fe2++2H2O
������Һ�õ���ˮ����ͭ��ʵ�������
���ȡ�����
���ȡ�����
��
��2���������������������Ṥҵβ���еĶ�������ͬʱ�Ƶ�����泥���Ҫ�Ĺ���������ͼ��ʾ��
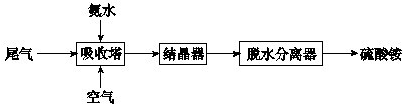
���������з�����Ӧ�Ļ�ѧ����ʽ��
4NH3?H2O+2SO2+O2�T2��NH4��2SO4+2H2O
4NH3?H2O+2SO2+O2�T2��NH4��2SO4+2H2O
��
�������ݱ���������������Һ��pH��5.5��6.0֮�䣬����Ч�ʽϸߣ�������һ��������β��ʱ��������Һ��pH�ķ�����
���ڰ�ˮ������
���ڰ�ˮ������
��





 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�