

| ||
| ���� |
| ||
| ���� |
| ||
| ���� |

| ʵ���� | ��Ʒ�����Լ� | �������� �����mL�� |
| 1 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 33.45 |
| 2 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 33.55 |
| 3 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 33.50 |
| 4 | 10.00mL����ˮ��0.2g������20mLŨ���� | 1.50 |
| 33.45ml+33.55ml+33.50ml |
| 3 |
| 0.03200ml��0.1000mol/L |
| 2mol |
| 44.8mg |
| 10ml |


 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ||
| ���� |
| ||
| ���� |
| ||
| ���� |
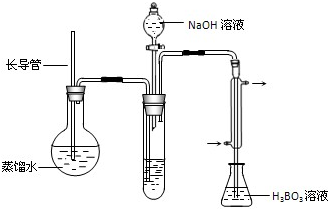
| ʵ���� | ��Ʒ�����Լ� | �������� �����mL�� |
| 1 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 33.45 |
| 2 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 33.55 |
| 3 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 33.50 |
| 4 | 10.00mL����ˮ��0.2g������20mLŨ���� | 1.50 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ר���� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
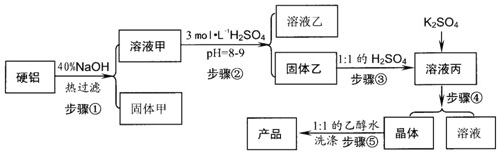
��13�֣�Ӳ�����ϣ���90%����2.5%þ��7.5%ͭ������ȡ����KAI(S04)2?12H20,ij̽��С�����������ʵ�顣

��ش��������⣺
��1)д������ڵ����ӷ���ʽ����������������pH��ֽ�ⶨ��ҺpHֵ�Ĺ��̣������ߡ�
��2)����ܰ��������������ڣ��ֱ�����������������������������
(3������ݲ�ֱ����ˮϴ��ԭ������������ϴ�ӵIJ�������������
(4)���˽��飺ֱ�ӽ�Ӳ������ϡH2S04�У����ˣ���Һ�м�����K2S04�õ���Һ����
�ٽ��к���ʵ�顣�������۴˽���Ŀ�������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 (8��)����Ӳ�����ϣ���90%����2.5%þ��7.5%ͭ�������ã�ij̽��С�����������ʵ�顣
(8��)����Ӳ�����ϣ���90%����2.5%þ��7.5%ͭ�������ã�ij̽��С�����������ʵ�顣
�Իش��������⣺
��1��д������A��һ����; �� ��д������B�Ļ�ѧʽ �� ��
��2��д����Ӧ�١��ڡ��۵����ӷ���ʽ��
�� �� �� �� �� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com