
50ml0.50mol��L-1������50mL0.55mol��L-1NaOH��Һ������ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ�����зų��������ɼ����к��ȡ�

�ش��������⣺
��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�������
�� ��װ���л����ڵ�2�������Ǣ�__
�� ���ִ�����������¶ȶ���________�����������С������Ӱ�족������õ��к��Ƚ�____________���ƫ����ƫС������Ӱ�족����
��2����ʵ������У���ͬѧ��Ҫ�ⶨ����¼��ʵ���������� ������ţ���
A�������Ũ��
B��������¶�
C������������Һ��Ũ��
D������������Һ���¶�
E��ˮ�ı�����
F����Ӧ������Һ����ֹ�¶�
��3��ʵ���и���60mL0��50mol��L-1�����50mL0��55mol��L-1NaOH��Һ���з�Ӧ����������ȷ��ʵ�������ȣ����ų������� �����ȡ�����ȡ����������к��� �����ȡ�����ȡ�����
��4������ͬŨ�Ⱥ�����İ�ˮ����NaOH��Һ��������ʵ�飬��õ��к�����ֵ�� �����ƫ��ƫС������Ӱ�족����
��1�����β�������� �����С�ձ�δ����ձ�������ƽ���ձ���δ������ֽ��
��С ƫС
��2��BDF��2�֣�
��3������� ���
��4��ƫС
��������
�����������1������IJ�������Ϊ���β����������������ʹ��Ͼ��ȣ���Ӧ�ӿ죬�Ҽ���������ɢʧ�������ʵ���ʱ���������ڴ��ձ��ײ�������ĭ���ϣ���ֽ������ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ���ϣ���ֽ�������Ѵﵽ���¡����ȡ�����ʵ������е�����ɢʧ���ʸ�ʵ��װ�ûᵼ������¶ȶ�����С�������к���ƫС��
��2������Ҫ��¼������Ϊ��ʼ�¶ȣ���������¶ȡ�����������Һ���¶ȣ�Ȼ��ȡ��ƽ��ֵ������ֹ�¶ȣ�����Ӧ������Һ����ֹ�¶�
��3������ȣ���Ϊ�ᡢ����кͷ�Ӧ�ų����������ᡢ��������йأ�
��ȣ��к�����ָ�������кͷ�Ӧ����lmol H2O���ų�������,�����ᡢ���������
��4���ð�ˮ�����������ƣ���Ϊ��ˮΪ�������룬������Ҫ���ȣ��ʻ�ʹ��õ��к�����ֵƫС��
���㣺�к��ȵ�֪ʶ
���������⿼�����к��Ȳⶨ��װ�ü��������裬��ǿα���֪ʶ�����ܺܺõĽ�����ѶȲ���


 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д� Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
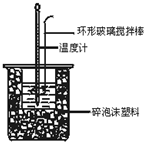 �к��Ȳⶨʵ���У���50mL0.50mol/L�����50mL0.55mol/LNaOH����ʵ�飬����˵������ȷ���ǣ�������
�к��Ȳⶨʵ���У���50mL0.50mol/L�����50mL0.55mol/LNaOH����ʵ�飬����˵������ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 50ml0.50mol?L-1������50mL0.55mol?L-1NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ�����зų��������ɼ����к��ȣ��ش��������⣺
50ml0.50mol?L-1������50mL0.55mol?L-1NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ�����зų��������ɼ����к��ȣ��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ����ǰ���� | ����ǰ���� | ���Ⱥ����� |
| W1�������� | W2������+���壩 | W3������+��ˮ����ͭ�� |
| 160(W2-W3) |
| 18(W3-W1) |
| 160(W2-W3) |
| 18(W3-W1) |
| 0.418��2.35 |
| 0.025 |
| 0.418��2.35 |
| 0.025 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com