
 ��ϩ��( )��һ����Ҫ���л��ϳ�ԭ�ϡ�
��ϩ��( )��һ����Ҫ���л��ϳ�ԭ�ϡ�
��1����ϩ���к��еĹ����ŵĻ�ѧ������
��2��0.3mol��ϩ����11.5g�����Ʒ�Ӧ�������ɱ�״���µ�����_________ L��
��3��д����ϩ������ˮ��Ӧ�Ļ�ѧ����ʽ____________________________
��4����ϩ����CH3 CO 18OH����������Ӧ�Ļ�����ʽΪ��
_______________________ _________
������Ӧ���ɵIJ�����һ�������¿��Է����Ӿ۷�Ӧ�õ��߷��ӻ������ṹ��ʽΪ
________
(5)��Ʒ����ñ�ϩ���ϳɱ�ϩ��������ո���д���������漰�����л�ѧ��Ӧ����ʽ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪H2A��ˮ�д�������ƽ�⣺H2AH����HA����HA��H����A2�����ش��������⣺
(1)NaHA��Һ________(������ԡ������Լ��ԡ����������ԡ����������ȷ����)��ԭ����___________________________________________ _______________________________________________________________��
(2)ij�¶��£���0.1 mol·L��1��NaHA��Һ�е���0.1 mol·L��1 KOH��Һ�����ԣ���ʱ��Һ��������ʾ��ϵһ����ȷ����________��
A��c(H��)·c(OH��)��1��10��14
B��c(Na��)��c(K��)��c(HA��)��2c(A2��)
C��c(Na��)��c(K��)
D��c(Na��)��c(K��)��0.05 mol·L��1
(3)��֪�����£�H2A�ĸ���(CaA)������Һ�д���ƽ�⣺CaA(s)Ca2��(aq)��A2��(aq)����H��0��
���¶�����ʱ��Ksp________(���������С�����䡱��ͬ)��
�ڵμ�����Ũ���ᣬc(Ca2��)________��ԭ����__________________________ _____________________________________________________________________________________________(�����ֺ����ӷ���ʽ˵��)��
(4)����CaA����Һ�м���CuSO4��Һ������һ�ֺ�ɫ�������ʣ�д���ù����з�Ӧ�����ӷ���ʽ__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ȡ0.1 mol Cu�ֱ�Ͷ��������������������Һ��(��Ҫʱ���Լ���)����Ũ�����Ũ�����ϡ�����ϡ���ᣬ��ַ�Ӧ��õ����������(��ͬ������)�ɴ�С��˳����(����)
A���٢ڢۢ� B���ڢ٢ۢ� C���ڢܢ٢� D���ۢܢ٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з����к�������̼ԭ�ӵ���( )
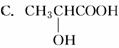 A��CF2Cl2 B��CH3CH2OH D��CH2===CH��COOH
A��CF2Cl2 B��CH3CH2OH D��CH2===CH��COOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʼ����ϩ(Pterodactyladiene)��״����һֻչ�������������ʽ�ṹ��ʾ���£�����R1��R2Ϊ���������������й�ʼ����ϩ��˵������ȷ���� �� ��)
A��ʼ����ϩ����ϩ��Ϊͬϵ��
B����R1��R2���������仯ѧʽΪC12H16
C����R1��R2��������ʼ����ϩ��һ�ȴ�����3��
D��ʼ����ϩ����ʹ���Ը��������Һ��ɫ��Ҳ��ʹ��ˮ��ɫ��������Ӧ�ķ�Ӧ��������ͬ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֬������Ҫʹ�������ʹӻ�������ַ��룬�ɲ��âٷ�Һ�������۹��ˡ����������������е�____________(�����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ϻ���ʦ��Ԣ�����ʩ���д���ʹ�õľ۰���ȼ���йأ��ٴα���NHCO(�۰���)��ĭ���²��ϵ���ȼװ�����Ѿ���Ϊ��ɻ��ֵ�������ס������й�˵����ȷ����(����)
A���۰������²���������
B���۰������ڼӾ��߷��Ӳ���
C���۰������ڴ�����
D���۰�������û�й̶����۵�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ����(����)
A����ϵͳ��������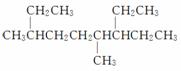 ������Ϊ4,7����3�һ�����
������Ϊ4,7����3�һ�����
B���Է���������ԭ�ӹ�ƽ��
C������( )��������[CH2OH(CHOH)4CHO]��Ԫ�������ͬ����ѧʽ��ΪC6H12O6������Cm(H2O)n��������Ƕ������������
)��������[CH2OH(CHOH)4CHO]��Ԫ�������ͬ����ѧʽ��ΪC6H12O6������Cm(H2O)n��������Ƕ������������
D��1 mol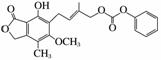 �������4 mol NaOH��ȫ��Ӧ
�������4 mol NaOH��ȫ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ҫ��ش��������⣺
(1) 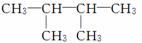 ��ϵͳ����Ϊ________��
��ϵͳ����Ϊ________��
(2)3��2��ϩ�Ľṹ��ʽΪ________��
(3) �ķ���ʽΪ________��
�ķ���ʽΪ________��
(4)ij���ķ���ʽΪC4H4�����Ǻϳ����м��壬���ж���ͬ���칹�塣
����д������һ����ʽ�ṹ��ͬ���칹��Ľṹ��ʽ____________��
������һ��ͬ���칹�壬ÿ��̼ԭ�Ӿ��ﱥ�ͣ���̼��̼�ļн���ͬ���÷�����̼ԭ���γɵĿռ乹��Ϊ____________�Ρ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com