
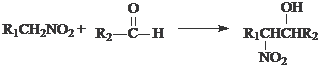 ����R1CH2NO2��������Ϊȩ����Ҳ�ܷ������Ʒ�Ӧ��
����R1CH2NO2��������Ϊȩ����Ҳ�ܷ������Ʒ�Ӧ��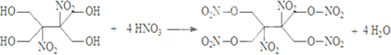

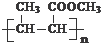 �����ϳɹ��������Լ���ѡ���ϳ�·������ͼʾ�����£�CH3CH2OH
�����ϳɹ��������Լ���ѡ���ϳ�·������ͼʾ�����£�CH3CH2OH| Ũ���� |
| 170�� |
| Br |
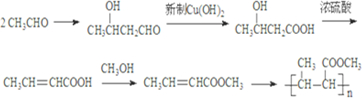

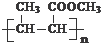 ����Ӧ������CH3CH=CHCOOCH3���������Ʒ��ƶϺϳ�·�ߣ�
����Ӧ������CH3CH=CHCOOCH3���������Ʒ��ƶϺϳ�·�ߣ� ��
�� ��
��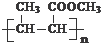 ����Ӧ������CH3CH=CHCOOCH3������CH3CH=CHCOOH��CH3OH���ɣ�
����Ӧ������CH3CH=CHCOOCH3������CH3CH=CHCOOH��CH3OH���ɣ� ��
�� ��
��

 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
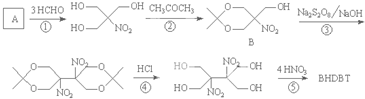
(1)A����Է���������__________________________��
(2)B�Ľṹ��ʽ��____________________________________________��
(3)C���ܵļ��ֽṹ��ʽ��__________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1������A����Է���������?
��2��д��������B�Ľṹʽ��?
��3��д��������C�Ľṹʽ��?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009-2010ѧ��������ѧ��һ��ѧ����ĩ���Ի�ѧ�����ƣ� ���ͣ�ѡ����
���ࡢ֬���͵�������ά����������������������Ӫ�����ʣ�����������ȷ���ǣ� ��
A�����ۡ���ά��û����ζ����˲���������
B����֬������������֬��ˮ�ⷴӦ��������Ӧ
C���������ܷ���������Ӧ��ˮ�ⷴӦ
D��Ũ���ὦ��Ƥ���ϣ�ʹƤ���ʻ�ɫ������Ũ����͵����ʷ�������ɫ��Ӧ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com