
��̼���ú�������Դ��������Դ��ú�����г����о���ͬ�¶��µ�ƽ�ⳣ����Ͷ�ϱȼ���ֵ�����⡣
��֪��CO(g) + H2O(g) H2(g) + CO2(g)��ƽ�ⳣ�����¶ȵı仯���±���
H2(g) + CO2(g)��ƽ�ⳣ�����¶ȵı仯���±���
|
�¶�/�� |
400 |
500 |
850 |
|
ƽ�ⳣ�� |
9.94 |
9 |
1 |
��ش��������⣺
��1����������Ӧ������ ��Ӧ������ȡ������ȡ�����
��2��850��ʱ�����Ϊ10L��Ӧ���У�ͨ��һ������CO��H2O��g������������Ӧ��CO��H2O(g)Ũ�ȱ仯����ͼ����0��4 min��ƽ����Ӧ����v(CO)��______ mol/(L��min)��
t1��ʱ����Ũ�ȣ�mol/L���ı仯

|
ʱ �䣨min�� |
CO |
H2O |
CO2 |
H2 |
|
0 |
0.200 |
0.300 |
0 |
0 |
|
2 |
0.138 |
0.238 |
0.062 |
0.062 |
|
3 |
C1 |
C2 |
C3 |
C3 |
|
4 |
C1 |
C2 |
C3 |
C3 |
|
5 |
0.116 |
0.216 |
0.084 |
|
|
6 |
0.096 |
0.266 |
0.104 |
|
(3) t1��(����850��)ʱ������ͬ�����з���������Ӧ�������ڸ����ʵ�Ũ�ȱ仯���ϱ���
�ٱ���3 min��4 min֮�䷴Ӧ����_____״̬��C1��ֵ_____0.08 mol/L (����ڡ�С�ڻ����)��
�ڷ�Ӧ��4 min��5 min��ƽ�����淽���ƶ������ܵ�ԭ����____(��ѡ)������5 min��6 min֮����ֵ�����仯�����ܵ�ԭ����______(��ѡ)��
A������ˮ���� B�������¶� C��ʹ�ô��� D����������Ũ��
��4������500��ʱ���У���CO��H2O����ʼŨ�Ⱦ�Ϊ0.020mol/L���ڸ������£�CO�����ת����Ϊ�� ��
��5������850����У�����ʼʱCO��H2O(g)��Ϊ5mol��ˮ�������������ΪX��ƽ��ʱCOת����ΪY�����Ƶ�Y��X�仯�ĺ�����ϵʽΪ ��
(6) ��ҵ������N2��H2���Ժϳ�NH3��NH3�ֿ��Խ�һ���Ʊ�����(N2H4)�ȡ���֪��
N2(g) + 2O2(g) ��2NO2(g) ��H = +67.7 kJ��mol��1
N2H4(g) + O2(g) ��N2(g) + 2H2O(g) ��H = ��534.0 kJ��mol��1
NO2(g)
 1/2N2O4(g)
��H = ��26.35 kJ��mol��1
1/2N2O4(g)
��H = ��26.35 kJ��mol��1
��д����̬��������̬������������ȼ�����ɵ�������̬ˮ���Ȼ�ѧ����ʽ��
________________________________________________________��
��1������ ��2�֣� ��2��0.03 ��2�֣�
��3���� ƽ�� ���ڣ�ÿ��1�֣� �� D A��ÿ��1�֣�
��4��75% ��2�֣� ��5��Y=X��2�֣�
��6��2N2H4(g) + N2O4(g)��3N2(g) + 4H2O(g) ��H = ��1083.0 kJ��mol��1��3�֣�
����������1�����ݱ������ݿ�֪�������¶ȵ����ߣ�ƽ�ⳣ����С��˵�������¶ȣ�ƽ�����淴Ӧ�����ƶ����������Ӧ�Ƿ��ȷ�Ӧ��
��2������ͼ���֪����0��4 minCO��Ũ�ȼ�����0.2mol/L��0.08mol/L��0.12mol/L�����Է�Ӧ������0.12mol/L��4min��0.03mol/(L��min)��
��3������3 min��4 min֮�䣬���ʵ�Ũ�Ȳ��ٷ����仯�����Է�Ӧ�ﵽƽ��״̬����������Ӧ�Ƿ��ȷ�Ӧ�����������¶ȣ�ƽ�����淴Ӧ�����ƶ������ƽ��ʱC1����0.08 mol/L��
������ˮ������Ũ�Ȼ��¶�ƽ�ⶼ������Ӧ�����ƶ�����������Ӱ��ƽ��״̬����������Ũ��ƽ�����淴Ӧ�����ƶ���D��ȷ��5min��6minʱ��CO��Ũ�Ƚ��ͣ�ˮ������������Ũ����������ƽ��������Ӧ�����ƶ�����˸ı������������ˮ������Ũ�ȣ���ѡA��
��4��
CO(g) + H2O(g) H2(g)
+ CO2(g)
H2(g)
+ CO2(g)
��ʼ����mol�� ��5��5X�� 5X 0 0
ת������mol�� ��5��5X��Y ��5��5X��Y ��5��5X��Y ��5��5X��Y
ƽ������mol����5��5X��5Y��5XY����5X��5Y��5XY�� ��5��5X��Y ��5��5X��Y
������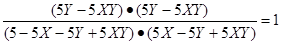
���Y��X
����CO��ת������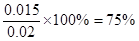
��5��
CO(g) + H2O(g) H2(g)
+ CO2(g)
H2(g)
+ CO2(g)
��ʼŨ�ȣ�mol/L�� 0.020 0.020 0 0
ת��Ũ�ȣ�mol/L�� x x x x
ƽ��Ũ�ȣ�mol/L�� 0.02��x 0.02��x x x
��6�������˹���ɵ�Ӧ�á�������֪��Ӧ��֪���ڡ�2���٣��ۡ�2���õ�2N2H4(g) + N2O4(g)��3N2(g) + 4H2O(g) �����Է�Ӧ�ȡ�H����534.0 kJ��mol��1��2��67.7 kJ��mol��1��26.35 kJ��mol��1��2�� ��1083.0 kJ��mol��1��


 ������ҵ��ͬ����ϰ��ϵ�д�
������ҵ��ͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?����һģ����̼���ú�������Դ��������Դ��ú�����г����о���ͬ�¶��µ�ƽ�ⳣ����Ͷ�ϱȼ���ֵ�����⣮
��2012?����һģ����̼���ú�������Դ��������Դ��ú�����г����о���ͬ�¶��µ�ƽ�ⳣ����Ͷ�ϱȼ���ֵ�����⣮| �¶�/�� | 400 | 500 | 850 |
| ƽ�ⳣ�� | 9.94 | 9 | 1 |
| ʱ�䣨min�� | CO | H2O | CO2 | H2 |
| 0 | 0.200 | 0.300 | 0 | 0 |
| 2 | 0.138 | 0.238 | 0.062 | 0.062 |
| 3 | c1 | c2 | c3 | c3[m] |
| 4 | c1 | c2 | c3 | c3 |
| 5 | 0.116 | 0.216 | 0.084 | |
| 6 | 0.096 | 0.266 | 0.104 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �¶�/�� | 400 | 427 | 700 | 800 |
| ƽ�ⳣ�� | 9.94 | 9 | b | 0.64 |
| ��Ӧʱ��/min | n��CO��/mol | H2O/mol |
| 0 | 1.20 | 0.60 |
| t1 | 0.80 | |
| t2 | 0.20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �¶�/�� | 400 | 500 | 850 |
| ƽ�ⳣ�� | 9.94 | 9 | 1 |
| ʱ �䣨min�� | CO | H2O | CO2 | H2 |
| 0 | 0.200 | 0.300 | 0 | 0 |
| 2 | 0.138 | 0.238 | 0.062 | 0.062 |
| 3 | C1 | C2 | C3 | C3 |
| 4 | C1 | C2 | C3 | C3 |
| 5 | 0.116 | 0.216 | 0.084 | |
| 6 | 0.096 | 0.266 | 0.104 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭�ٺ��а��Ƹ���ѧ�߶���ѧ�����п��Ի�ѧ������������ ���ͣ������
��ÿ��1�֣���8�֣���̼���ú�������Դ��������Դ��ú�����г����о���ͬ�¶��µ�ƽ�ⳣ����Ͷ�ϱȼ���ֵ�����⡣
��֪��CO(g)��H2O(g) H2(g)��CO2(g)��ƽ�ⳣ�����¶ȵı仯���±���
H2(g)��CO2(g)��ƽ�ⳣ�����¶ȵı仯���±���
| �¶�/�� | 400 | 500 | 850 |
| ƽ�ⳣ�� | 9.94 | 9 | 1 |

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com