����⣺Q��W��X��Y��Z��5�ֶ�����Ԫ�أ�W��Y��X��Y��ɵĻ������ǻ������ų��Ĵ�����Ⱦ��������ų��Ĵ�����Ⱦ�ﳣ������CO��NO��W��X��Yԭ����������������WΪCԪ�أ�XΪNԪ�أ�YΪOԪ�أ�Q��W��ɵĻ������Ǿ�������ЧӦ�����壬ΪCH
4���壬��QΪHԪ�أ�Y��Z���γ�ԭ�Ӹ�����Ϊ1��1��1��2���������ӻ����ӦΪNa
20��Na
2O
2���ֻ������ZΪNaԪ�أ���QΪHԪ�أ�WΪCԪ�أ�XΪNԪ�أ�YΪOԪ�أ�ZΪNaԪ�أ�
��1��WΪCԪ�أ���2�����Ӳ㣬����������Ϊ4��λ�����ڱ��ڶ�����IVA�壻Z
2Y��Na
2O�����������������ӹ��ɣ�����ʽ��
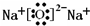
��
�ʴ�Ϊ���ڶ�����IVA�壻
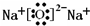
��
��2����ҵ�ϳ�NH
3�������С�ķ��ȷ�Ӧ�����д�ʩ�У����ܼӿ췴Ӧ���ʣ��������ԭ��ת���ʵ���
a�������¶ȣ���Ӧ�ӿ죬ƽ�������ȷ����ƶ��������淴Ӧ�ƶ���ԭ��ת���ʽ��ͣ���a�����ϣ�
b���������������Ӧ���ʣ�ƽ�ⲻ�ƶ���ԭ��ת���ʲ��䣬��b�����ϣ�
c����XQ
3��ʱ�����ȥ����Ӧ���ʼ�С��ƽ��������Ӧ�ƶ���ԭ��ת��������c�����ϣ�
d������Ӧ��ϵ��ѹǿ����Ӧ���ʼӿ죬ƽ��������Ӧ�ƶ���ԭ��ת��������d���ϣ�
��ѡ��d��
��3��2.24L����״����NH
3Ϊ0.1mol��200mL1mol/L HNO
3��Һ����HNO
30.2mol��������������Һ���գ���Һ�൱�ں���0.1molHNO
3��0.1molNH
4NO
3��ϣ�笠�����ˮ�ⲻ����Һ�����ԣ�������Һ������Ũ�ȴӴ�С��˳����c��NO
3-����c��H
+����c��NH
4+����c��OH
-����
�ʴ�Ϊ��c��NO
3-����c��H
+����c��NH
4+����c��OH
-����
��4����ͼ��֪�����Ӵ�a��������a��Ϊԭ��ظ�������������������Ӧ��CH
4O�ڸ����Ϸŵ磬�缫��ӦʽΪCH
3OH-6e
-+8OH
-=CO
32-+6H
2O��
�ʴ�Ϊ��CH
3OH-6e
-+8OH
-=CO
32-+6H
2O��
��5����֪����C��s��+O
2 ��g��=CO
2��g����H=-393.5kJ/mol��
��CO��g��+
O
2 ��g��=CO
2��g����H=-283.0kJ/mol��
�ɢ�-�ڵã�C��s��+
O
2 ��g��=CO��g����H=-110kJ/mol��
24gC�����ʵ���Ϊ2mol����һ������O
2��Ӧ����ֻ���ɶ�����̼���ų�����Ϊ393.5kJ/mol��2mol=787kJ����ֻ����һ����̼���ų�����Ϊ110kJ/mol��2mol=220kJ��ʵ�ʷų�����362.5kJ�������ɶ�����̼��һ����̼�������ɶ�����̼xmol��һ����̼ymol������x+y=2��393.5x+110y=362.5�����x=0.5��y=1.5������n��CO
2����n��CO��=1��3��
�ʴ�Ϊ��n��CO
2����n��CO��=1��3��
��6��X��Z��ɵ�һ�����ӻ��������ˮ��Ӧ�������ּ�û�����ΪNa
3N���÷�Ӧ�Ļ�ѧ����ʽ��Na
3N+4H
2O=3NaOH+NH
3?H
2O��
�ʴ�Ϊ��Na
3N+4H
2O=3NaOH+NH
3?H
2O��

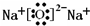
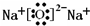
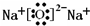 ��
��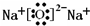 ��
��


 ��2011?������һģ������ͼ��ʾ��װ���л�����ͨ������X�����رջ���K����Ʒ����Һ�ޱ仯������ʯ��ˮ����ǣ�������K����Ʒ����Һ��ɫ��X��Y�����ǣ�������
��2011?������һģ������ͼ��ʾ��װ���л�����ͨ������X�����رջ���K����Ʒ����Һ�ޱ仯������ʯ��ˮ����ǣ�������K����Ʒ����Һ��ɫ��X��Y�����ǣ�������