
ʵ������Ҫ0��2mol/L CuSO4��Һ250ml��ʵ���ҿ��ṩ������Һ���Լ��У�����ɫ��������(CuSO4��5H2O) ��4mol/L CuSO4��Һ
(1)���۲��ú����Լ��������ƣ�ʵ������õ��IJ����������ձ�������������ͷ�ι��⣬���ٻ���Ҫ��һ��������________����ʹ�ø�����ǰ������еIJ����� ��
(2)���õ�������������ƣ�����������ƽ��ȡCuSO4�� 5H2O������Ϊ________g�������4mol/L��CuSO4��Һϡ�����ƣ�������Ͳ��ȡ___________ml4mol/L CuSO4��Һ��
(3)ʵ������4mol/L������ͭ��Һϡ��������Һ�����ʵ�鲽���У�
������ȷ�IJ���˳��Ϊ
�����ձ��м���Լ100mlˮ���г���ϡ�ͣ���ȴ������
������Ͳ��ȡһ�����4mol/L ������ͭ��Һ��һ�ձ���
�ۼ�������4mol/L ����ͭ��Һ�����
�ܽ���Һ�ߵ�ҡ�Ⱥ�ת�����Լ�ƿ
�ݼ�ˮ��Һ��������ƿ1-2cm�����ý�ͷ�ιܽ��ж���
��ϴ���ձ��Ͳ�����2-3�β���ϴ��Һע������ƿ������ҡ������ƿ��ʹ��Һ��Ͼ���
�߽���Һת��������ƿ
(4)������Һ�����У������������������Խ���к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족��
�������ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ��ټ�ˮ���̶���
B������ʱ���ӿ̶���
C������ƿδ���T����������Һ
��5��ʵ��������õ�����ˮ����ͼΪʵ������ȡ����ˮ��װ��ʾ��ͼ��ͼ�е��������ԵĴ����� ______________________________��_______________________________��ʵ��ʱA�г�������������ˮ�⣬�����������___ ____���������Ƿ�ֹ���С�
��1��250mL����ƿ��1�֣��� ����Ƿ�©Һ��1�֣���
��2��12��5��1�֣��� 12��5��1�֣���
��3��???????��2�֣���
��4��ƫ�ͣ�1�֣��� ƫ�ߣ�1�֣��� ��Ӱ�죨1�֣���
��5���¶ȼƵ�ˮ����Ӧ��������ƿ֧�ܿڴ���1�֣��� ������ӦΪ�¿ڽ�ˮ���Ͽڳ�ˮ����1�֣��� ���Ƭ����ʯ����1�֣���
�������������
��1��ʵ������Ҫ0��2mol/L CuSO4��Һ250ml��������Ҫ250mL����ƿ��
ʹ�ø�����ǰ������еIJ���Ҫ����Ƿ�©Һ������Ӱ��ʵ�����ݡ�
CuSO4�����ʵ���n=cV=0��25L��0��2mol?L-1=0��05mol��CuSO4?5H2O�����ʵ�������CuSO4�����ʵ���������CuSO4?5H2O������0��05mol��250g/mol=12��5g��
�����4mol/L��CuSO4��Һϡ�����ƣ�������Ͳ��ȡ4mol/L CuSO4��Һ�����Ϊ��0��05mol/(4mol/L )=0��0125L=12��5mL��
��3��???????
��4��A�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ�������Һ������ƿ����ƿ��֮�䣬�ټ�ˮ���̶��ߣ�������Һ�����ƫ����ҺŨ��ƫС��
B������ʱ���ӿ̶��ߣ�����������Һ�����ƫ����ҺŨ��ƫС��
C����Һ�������ˮ���ݣ�����ƿδ���T����������Һ����������ҺŨ����Ӱ�죻
��5���¶ȼƵ�ˮ����Ӧ��������ƿ֧�ܿڴ���������ӦΪ�¿ڽ�ˮ���Ͽڳ�ˮ�� ���Ƭ����ʯ����
���㣺һ�����ʵ���Ũ�ȵ���Һ������


 ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ��һ����ʵ��Ϊ���Ŀ�ѧ����ѧʵ����ѧϰ̽���������ʵĻ�������֮һ��
��1�������й�������ȷ����__________����д��ţ�
a��ʹ��������ƽ�ĵ�һ�������ǽ��������������̶ȴ�
b�����˲��������У�Ϊ�ӿ�����ٶȿ��ò�������©���е���Һ���н���
c����Ũ��������ϡ��Һʱ������Ͳ�к�ϡ��Ҫ��ȴ��������ת�Ƶ�����ƿ��
d��������ƿ������Һʱ�����ݺ�ҡ��Һ���½����ټ�����ˮ���̶��ߴ���������ҺŨ��ƫ��
��2�������������壺H2��O2��NH3��SO2��NO2��NO������������ͼ��ʾװ�ý����ռ���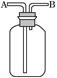
���������B�ڽ��룬���ռ���������_______________��
��������ƿ��ע��ˮ��������Ӧ�ô�______����д��A����B�����ڽ��룬�����ռ���������_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
K3[Fe��C2O4��3]��3H2O[���������������ؾ���]������ˮ���������Ҵ�������Ϊ�л���Ӧ�Ĵ�����ʵ���ҿ�����мΪԭ���Ʊ�����ط�Ӧ�������¡���ش��������⣺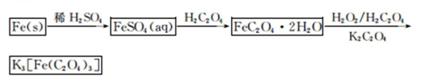
��1����м�г�����Ԫ�أ�������Ʊ�FeSO4ʱ������ж���H2S���壬�������������������Һ���ա���������װ����ȷ���� ������ţ���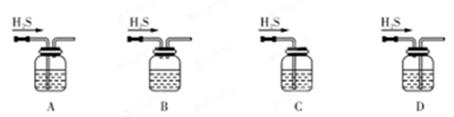
��2���ڵõ���FeSO4��Һ�������������H2 SO4�ữ��Ŀ���� ���õ�K3[Fe(C2O4)3]��Һ�����Ҵ���Ŀ���� ��
��3�������������ᾧˮ��ͨ�������������ⶨ����Ҫ�����У��ٳ����������ں������ѽᾧˮ������ȴ���ܳ��������ظ��ڡ��������أ����㡣
����ݵ�Ŀ���� ��
��4��C2O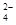 �ɱ�����KMnO4��Һ����ΪCO2���壬��ʵ�������K3[Fe��C2O3��3]��3H2O�����ⶨ����KMnO4����Һ�ζ���
�ɱ�����KMnO4��Һ����ΪCO2���壬��ʵ�������K3[Fe��C2O3��3]��3H2O�����ⶨ����KMnO4����Һ�ζ���
��д���ζ������з�����Ӧ�����ӷ���ʽ ��
�����еζ�������ʹ�ζ����ƫ�ߵ��� ������ţ���
| A���ζ���������ˮϴ�Ӻ�����װ���Һ |
| B����ƿ��װ����Һǰδ�ô���Һ��ϴ |
| C���ζ�ǰ�ζ��ܼ��촦�����ݣ��ζ���������ʧ |
| D����ȡ��Һ���ʱ���ζ�ǰ���Ӷ������ζ����Ӷ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Ϊ̽��ij��̼�Ͻ���Ũ�����ڼ��������µķ�Ӧ�IJ��ֲ��ﲢ�ⶨ��̼�Ͻ�����Ԫ�ص���������,ij��ѧ�С���������ͼ��ʾ��ʵ��װ��,���������ʵ��̽����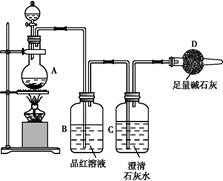
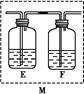
(1)��Բ����ƿ�м���m g��̼�Ͻ�,���������Ũ����,δ��ȼ�ƾ���ǰ,A��B������������,��ԭ����:�ٳ�����,Fe��Ũ�����жۻ�;��������������������
(2)��Ӧһ��ʱ���,��A���ݳ������������Ȼ�Ͽ�,����Ӧ�¶Ƚϸ���,�����ܵ�ԭ������ ��
(3)װ��B���������� ��
(4)��ͬѧ�۲쵽װ��C���а�ɫ��������,���ó���ʹ����ʯ��ˮ����ǵ������Ƕ�����̼��װ��A���ܲ���������̼�Ļ�ѧ����ʽΪ�� ��
(5)��ͬѧ��Ϊ��ͬѧ�Ľ����Ǵ����,����ΪΪ��ȷ�϶�����̼�Ĵ���,����װ��B-C֮������װ��M��װ��E��F��ʢ�ŵ��Լ��ֱ�����������������������������������������������ʵ���۲쵽װ��F�е��������� ����
(6)��Щͬѧ��Ϊ�Ͻ�����Ԫ�ص�������������KMnO4��Һ���ⶨ(5Fe2++Mn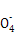 +8H+
+8H+ 5Fe3++Mn2++4H2O)��
5Fe3++Mn2++4H2O)��
�ⶨ��Ԫ������������ʵ�鲽������:
��.����ƿA�м�������Ļ�ԭ��ʹ��Һ�е�Fe3+��ȫת��ΪFe2+,����,�õ���ҺB;
��.����ҺBϡ��Ϊ250 mL;
��.ȡϡ��Һ25.00 mL,��Ũ��Ϊc mol��L-1������KMnO4��Һ�ζ�,���εζ�ʵ������KMnO4��Һ�����ƽ��ֵΪV mL��
�ٲ������,����ҺBϡ��Ϊ250 mL��Ҫ�õ��IJ����������ձ�������������ͷ�ι���,������Ҫ�õ���������������
�ڱ�ͬѧ��������еζ���ʽ(�г�����ʡ��),�����������������(����ĸ���)�� 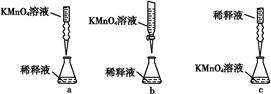
�۵ζ���������������(���Ҫ������Ҫ��)����ָʾ����
����̼�Ͻ�����Ԫ�ص���������Ϊ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ѧ��һ����ʵ��Ϊ�����Ŀ�ѧ��
��1��������������ȷ����______������ţ���
| A����Һ©�����ζ��ܺ�����ƿʹ��ǰ�������Ƿ�©ˮ |
| B������ˮ�����Һ©�����ټ������Ҵ�����������ã��ɴӵ�ˮ����ȡ�� |
| C���ྻ��������ʳ��ˮ�н���һ��ʱ�䣬�����������ݣ�˵�������������ⸯʴ |
| D����˿�������о���ȼ�գ��������䣬���ɺ�ɫ���� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ���ҳ����ü�ȩ��HCHO�����ⶨ(NH4)2SO4��Ʒ�е��������������䷴Ӧԭ��Ϊ��4NH4�� ��6HCHO =3H����6H2O��(CH2)6N4H�� �۵ζ�ʱ��1 mol (CH2)6N4H���� l mol H���൱��Ȼ����NaOH����Һ�ζ���Ӧ���ɵ��ᡣij��ȤС���ü�ȩ������������ʵ�飺
����I ��ȡ��Ʒ1��500 g��
����II ����Ʒ�ܽ����ȫת�Ƶ�250 mL����ƿ�У����ݣ����ҡ�ȡ�
����III ��ȡ25��00 mL��Ʒ��Һ��250 mL��ƿ�У�����10 mL 20�������Լ�ȩ��Һ��ҡ�ȡ�����5 min����1~2�η�̪��Һ����NaOH����Һ�ζ����յ㡣�����������������ظ�2�Ρ�
��1�����ݲ���III��գ�
�ټ�ʽ�ζ���������ˮϴ�Ӻ�ֱ�Ӽ���NaOH����Һ���еζ���������Ʒ�е�����������________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
����ƿ������ˮϴ�Ӻ�ˮδ��������ζ�ʱ��ȥNaOH����Һ�����_______(�ƫ����ƫС������Ӱ�족)
�۵ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲�____________��
A���ζ�����Һ��ı仯 B����ƿ����Һ��ɫ�ı仯
�ܵζ��ﵽ�յ�ʱ����__________________________________________________��
��2���ζ�������±���ʾ��
| �ζ� ���� | ������Һ����� /mL | ����Һ�����/mL | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| 1 | 25��00 | 1��02 | 21��03 |
| 2 | 25��00 | 2��00 | 21��99 |
| 3 | 25��00 | 0��20 | 20��20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ���Һϳ����������IJ������£�
��Բ����ƿ�ڼ����Ҵ���Ũ��������ᣬƿ����ֱ��װͨ����ȴˮ��������(ʹ��Ӧ��������������ΪҺ��������ƿ��)�����Ȼ���һ��ʱ�������װ�ý������õ������Ҵ��������ˮ�����������ֲ�Ʒ��
��ش��������⣺
��1������ƿ�г��˼������ᡢŨ������Ҵ��⣬��Ӧ�������Ƭ��Ŀ����__________________��
��2����Ӧ�м���������Ҵ���Ŀ����________________________________________________��
��3�����������ʵ�鲽���Ϊ��������ƿ���ȼ����Ҵ���Ũ���ᣬȻ��ͨ����Һ©���ߵμ����ᣬ��������������������������IJ��ʣ���ԭ����_____________________��
��4���������ֲ�Ʒ����������������Ҵ��Ļ�����ͼ�Ƿ��������������ͼ��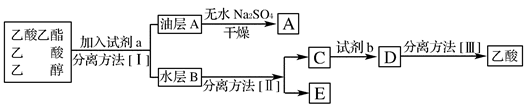
���Լ�a�ǣ�________�����뷽�����ǣ�________________________�����뷽�����ǣ�____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�Ա���ۣ���Ҫ�ɷ�ΪBaCO3������Ca2+��Fe2+��Fe3+��Mg2+�ȣ��Ʊ�BaCl2��2H2O���������£�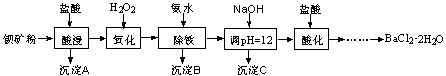
��1������������Ҫ��Ӧ�����ӷ���ʽΪ ��
��2������C����Ҫ�ɷ���Ca(OH)2�� ��
��ͼ��֪��Ϊ�˸��õ�ʹCa2+��������Ӧ��ȡ�Ĵ�ʩΪ ��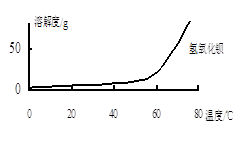
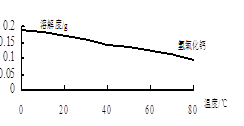
��3����BaSO4�������ⶨ��Ʒ���ȵIJ���Ϊ��
����1��ȷ��ȡ0.4��0.6 g BaCl2��2H2O����������100 ml ˮ��3 ml 2 mol��L-1��HCl��Һ�����ܽ⡣
����2���߽��裬����μ���0.1 mol��L-1 H2SO4��Һ��
����3����BaSO4������ ��ȷ������ȫ������
����4�����ˣ���0.01 mol��L-1��ϡH2SO4ϴ�ӳ���3~4�Σ�ֱ��ϴ��Һ�в���Cl��Ϊֹ��
����5�����۵��ij�����ֽ������ �У�����ɡ�̿�����һ�����800�����������ء���������BaCl2��2H2O��Ba2+�ĺ�����
�ٲ���3��ȱ�IJ���Ϊ ��
��������1��������Ʒ���٣����ڲ���4ϴ��ʱ������ɵ�Ӱ��Ϊ ��
�۲���5���ô�����������Ϊ ����ֽ�һ�ʱ����Ҫ���㣬����BaSO4�ױ�������̿��ԭ����BaS���÷�Ӧ�Ļ�ѧ����ʽΪ ��
����ͬѧ��Ϊ��K2CrO4����H2SO4��������Ч�����ã���˵��ԭ�� ��
[��֪��Ksp(BaSO4)=1.1��10-10 Ksp(BaCrO4)=1.2��10-10]
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����6����ɫ��Һ���Ҵ������ӡ�Na2CO3��Һ��AgNO3��Һ��KOH��Һ�������ᣬ���ֵ��Լ���
| A�����Ƽ���Cu(OH)2����Һ | B��FeCl3��Һ |
| C��BaCl2��Һ | D������KMnO4��Һ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com