
���� �����ܷ������������ķǽ�������A����Ӧ��DΪǿ�ᣬ���Ӧ�ķ�Ӧ����͵�Ԫ�ص�ת����
��1��A�ڳ�����Ϊ���壬B����ʹƷ����Һ��ɫ���д̼�����ζ����ɫ���壬��AΪSԪ�أ�BΪSO2��CΪSO3��DΪH2SO4��
��2����A�ڳ�����Ϊ���廯���C�Ǻ���ɫ�����壬��AӦΪNH3��BΪNO��CΪNO2��DΪHNO3��������ʵ����ʽ����⣮
��� �⣺�����ܷ���������������Ӧ�ķ�Ӧ����͵�Ԫ�ص�ת����
��1��A�ڳ�����Ϊ���壬B����ʹƷ����Һ��ɫ���д̼�����ζ����ɫ���壬��AΪSԪ�أ�BΪSO2��CΪSO3��DΪH2SO4��
��DΪH2SO4��D��Ũ��Һ��ľ̿��Ӧ�Ļ�ѧ��Ӧ����ʽΪC+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+2SO2��+2H2O���ʴ�Ϊ��H2SO4��
���ڹ�ҵ������SO2����Ĵ����ŷű���ˮ���պ��γ����������Ⱦ�˻������ʴ�Ϊ�����ꣻ
��B��C�ķ�Ӧ����ʽΪ2SO2+O2$\frac{\underline{����}}{��}$2SO3���ʴ�Ϊ��2SO2+O2$\frac{\underline{����}}{��}$2SO3��
��2����A�ڳ�����Ϊ���廯���C�Ǻ���ɫ�����壬��AӦΪNH3��BΪNO��CΪNO2��DΪHNO3��
��AӦΪNH3��BΪNO��A����O2��Ӧ����NO����Ӧ�Ļ�ѧ����ʽΪ��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
�ʴ�Ϊ��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
��CΪNO2��DΪHNO3����Ӧ�����ӷ���ʽΪ3NO2+H2O=2H++2NO3-+NO���ʴ�Ϊ��3NO2+H2O=2H++2NO3-+NO��
���� ���⿼��������ƶϣ���Ŀ�Ѷ��еȣ�����ע��������ʵ���ɫ�Լ���������������Ӧ������Ϊͻ�ƿڽ����ƶϣ�


 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д� Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
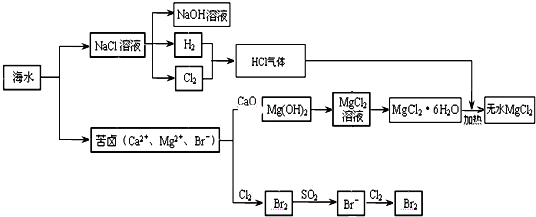
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ֻ�Т٢� | B�� | ֻ�Тڢ� | C�� | ֻ�Т٢ڢ� | D�� | �٢ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �TCH-CHO
�TCH-CHO +CO$��_{�⻯��}^{HCl}$
+CO$��_{�⻯��}^{HCl}$

 +CH3CHO$\stackrel{ϡ��}{��}$
+CH3CHO$\stackrel{ϡ��}{��}$ ��
��
 $��_{��Ӧ����1}^{G}$H$\stackrel{Ũ����}{��}$
$��_{��Ӧ����1}^{G}$H$\stackrel{Ũ����}{��}$ �Լ�G�Ľṹ��ʽ��CH3CH2CHO��
�Լ�G�Ľṹ��ʽ��CH3CH2CHO���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

 ��д���������ڷ��������A��ͬ���칹��
��д���������ڷ��������A��ͬ���칹�� ��
�� ��
�� ����������A��д�ṹ��ʽ��
����������A��д�ṹ��ʽ�� +O2$��_{��}^{Cu}$2
+O2$��_{��}^{Cu}$2 +2H2O����Ӧ������������Ӧ��C��E��Ӧ�Ļ�ѧ������
+2H2O����Ӧ������������Ӧ��C��E��Ӧ�Ļ�ѧ������ +NaOH $��_{��}^{ˮ}$
+NaOH $��_{��}^{ˮ}$ +NaCl��
+NaCl�� ����Ӧ������������Ӧ��
����Ӧ������������Ӧ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ũ�Ⱦ�Ϊ0.1mol•L-1��С�մ���Һ���ռ���Һ�������ϣ�2c��CO${\;}_{3}^{2-}$��+c��OH-��+c��HCO${\;}_{3}^{-}$��-c��H+��=0.1mol•L-1 | |
| B�� | Ũ�Ⱦ�Ϊ0.1mol•L-1�����������Һ������������Һ��������c��SO${\;}_{4}^{2-}$����c��Na+����c��NH${\;}_{4}^{+}$����c��H+����c��OH-�� | |
| C�� | pH=12�İ�ˮ��pH=2�������������c��Cl-����c��NH${\;}_{4}^{+}$����c��OH-����c��H+�� | |
| D�� | Ũ�Ⱦ�Ϊ0.1mol•L-1�Ĵ�����Һ������������Һ��������c��Na+��=c��CH3COO-����c��OH-��=c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
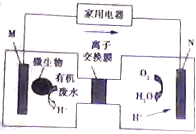 ��ý�屨��һ��������ˮ����װ����������װ�ÿ�����һ�����ォ�л����ˮ�Ļ�ѧ��ֱ��ת��Ϊ���ܣ���װ�õĹ�����ͼ��ʾ������˵������ȷ���ǣ�������
��ý�屨��һ��������ˮ����װ����������װ�ÿ�����һ�����ォ�л����ˮ�Ļ�ѧ��ֱ��ת��Ϊ���ܣ���װ�õĹ�����ͼ��ʾ������˵������ȷ���ǣ�������| A�� | װ�����·�м�ͷ�ķ�����������ķ��� | |
| B�� | ��װ��Ϊԭ��أ�����NΪ���� | |
| C�� | ��״���£�N�缫ÿ����11.2L����ʱ����4NA����ͨ�����ӽ���Ĥ | |
| D�� | ���л���ˮ�к��������ǣ���M�缫�����ĵ缫��ӦΪ��C6H12O6+6H2O-24e-�T6CO2+24H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������Ϊ60 | B�� | ������Ϊ27 | C�� | ���������Ϊ14 | D�� | ������Ϊ33 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com