
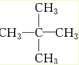
 ������Ϊ2��2-�������飨�����飩��
������Ϊ2��2-�������飨�����飩��
���� ��1��ϩ����±�ص��ʷ����ӳɷ�Ӧ�����ȴ��������ݼӳɷ�Ӧ��ʵ�����ش�
��2��������ͨʽΪCnH2n+2��������Է���������֪12n+2n+2=72����n=5���������ķ���ʽӦΪC5H12����������Ӧ���ɵ�һ�ȴ���ֻ��һ�֣�˵���ṹ�Գƣ�������ֻ����һ��H���Դ˽����⣻
��3���ٸ��ݼ���ʽ���ص�����ʾ����ÿ���۵���߶˵㴦��ʾ��һ��̼ԭ�ӣ�������ԭ�Ӳ����ļ���C��Hԭ�Ӳ���ʾ������д��ѧʽ��
�ڸ��ݽṹ��ʽ��C��H��O�ĸ�������д����ʽ��
���ڷ���ģ���и���ԭ�ӳɼ���Ŀ�жϣ�С�����ʾH�������ʾC�������ʾO��
��� �⣺��1��1molCH3CH2C��CH3��=CH2��1molBr2�����ӳɷ�Ӧ����1molCH3CH2CBr��CH3��CH2Br���ʴ�Ϊ��CH3CH2CBr��CH3��CH2Br��
��2��������ͨʽCnH2n+2������������Է�������Ϊ72�������У�12n+2n+2=72����14n=70�����n=5��������ʽΪC5H12����5��̼ԭ�ӵ�������һ��ȡ����ֻ��һ�֣�˵����������12����ԭ�ӵ�λ�þ��ǵ�Ч�ģ����ӽṹ�Գƣ��ṹ��ʽӦΪ ��Ϊ2��2-�������飨�����飩��
��Ϊ2��2-�������飨�����飩��
�ʴ�Ϊ��2��2-�������飨�����飩��
��3���ٸ��ݼ���ʽ����д�ص㣬��ṹ��ʽΪ��CH2=C��CH3��COOCH3���ʴ�Ϊ��CH2=C��CH3��COOCH3��
�ڸ��ݽṹ��ʽ��C��H��O�ĸ�������д����ʽ�����Է���ʽΪ��C10H16O���ʴ�Ϊ��C10H16O��
���ڷ���ģ���и���ԭ�ӳɼ���Ŀ�жϣ�С�����ʾH�������ʾC�������ʾO��������ṹ��ʽΪ��CH2=C��CH3��COOH���ʴ�Ϊ��CH2=C��CH3��COOH��
���� ������Ҫ�����˼���ʽ���ṹ��ʽ�����ģ�͵��ص㣬ץס���ص㼴��д�������ʽ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ľ�ң�����K2CO3��ˮ��Һ��������ϴ������ | |
| B�� | �Ҵ�������ͼ�ȩ�㷺Ӧ����ʳƷ�ӹ� | |
| C�� | �����ŷŵ�CO2�ܽ��͵��������ЧӦ | |
| D�� | ��ӦNH3��g��+HCl��g��=NH4Cl��s���������¿��Է����У���÷�Ӧ�ġ�H��0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��������ҽԺ���õ�����������������ǿ�ҵĸ�ʴ���ã��ܸ�ʴƤ�������ܣ���ʹ�����ʱ��ԣ����Ա���С��ʹ�ã���ѧ��ѧ����Ũ��ˮ���鱽�ӣ���д���÷�Ӧ�Ļ�ѧ����ʽ
��������ҽԺ���õ�����������������ǿ�ҵĸ�ʴ���ã��ܸ�ʴƤ�������ܣ���ʹ�����ʱ��ԣ����Ա���С��ʹ�ã���ѧ��ѧ����Ũ��ˮ���鱽�ӣ���д���÷�Ӧ�Ļ�ѧ����ʽ ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | �����м���С��109.5������Ϊ��ԭ�ӹ���ӻ�����Ϊsp2�ӻ� | |
| B�� | Cl�ĵ縺��ǿ��N�ĵ縺�� | |
| C�� | NCl3�����Ǽ��Է��� | |
| D�� | NBr3 ��NCl3�ӷ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Mg2+��OH-��NO3-��K+ | B�� | H+��HCO3-��Ca2+��NO3- | ||
| C�� | Cl-��OH-��H+��K+ | D�� | Cu2+��SO42-��Na+��Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ̼���ƣ����壩 | B�� | ˮ | C�� | �������Һ | D�� | CH3COONa�����壩 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | SiO2��H2SiO3 | B�� | Cu��OH��2��Fe��OH��3 | C�� | CaCO3��Na2CO3 | D�� | K2O��KOH |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com