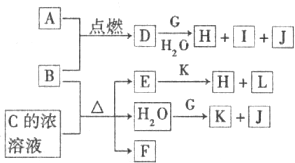
��2012?��ɽ��ģ��ͼ�У�A��LΪ�������ʻ�����ʵ�ˮ��Һ��B��A������ȼ�ղ����ػ�ɫ�̣�B��GΪ��ѧ��ѧ�г����Ľ������ʣ�E��ϡ��ҺΪ��ɫ��I����ɫ��ӦΪ��ɫ�����J��Ԫ��ԭ�Ӻ���ֻ��һ�����ӣ�FΪ��ɫ���д̼�����ζ�����壬����ʹƷ����Һ��ɫ��
�ش��������⣺
��1��AԪ�������ڱ��е�λ����
��3���ڢ�A��
��3���ڢ�A��
�����������ں��壩��K�����������Ļ�ѧ����
���Ӽ����ۼ������Թ��ۼ���
���Ӽ����ۼ������Թ��ۼ���
��
��2����D��ˮ��Һ��G��Ӧ�����ӷ���ʽΪ
Cu2++2H2O+2Na�TCu��OH��2��+2Na++H2��
Cu2++2H2O+2Na�TCu��OH��2��+2Na++H2��
��
��A��K����Ҫ�Ļ���ԭ�ϣ���ҵ��ͬʱ��ȡA���ʺ�K�ķ�Ӧ�Ļ�ѧ����ʽΪ
2NaCl+2H
2O
2NaOH+H
2��+Cl
2��
2NaCl+2H
2O
2NaOH+H
2��+Cl
2��
��
��3��������״����2.24L��Fͨ��150mL 1.0m1?L
-1��K��Һ�У���ַ�Ӧ�����Һ�����ԣ��������ʼ������ʵ����ֱ�Ϊ
Na2SO3Ϊ0.05mol��NaHSO3Ϊ0.05mol
Na2SO3Ϊ0.05mol��NaHSO3Ϊ0.05mol
��
��4����F���Ԫ����ͬ��һ��-2���������M��M������Ԫ�ص�������Ϊ4��3����֪1mol A�����뺬1mol M����Һ��ǡ����ȫ��Ӧ����Ӧʱ���۲쵽��dz��ɫ����������ȡ��Ӧ����ϲ���Һ���������ữ���Ȼ�����Һ���а�ɫ������������A�����뺬M����Һ��Ӧ�����ӷ���ʽΪ
Cl2+S2O32-+H2O�T2Cl-+2H++S��+SO42-
Cl2+S2O32-+H2O�T2Cl-+2H++S��+SO42-
��




 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
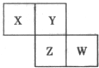 ��2012?��ɽ��ģ��X��Y��Z��W��Ϊ������Ԫ�أ����������ڱ������λ����ͼ��ʾ����Yԭ�ӵ��������������ڲ��������3��������˵������ȷ���ǣ�������
��2012?��ɽ��ģ��X��Y��Z��W��Ϊ������Ԫ�أ����������ڱ������λ����ͼ��ʾ����Yԭ�ӵ��������������ڲ��������3��������˵������ȷ���ǣ�������