
��12�֣���֪pHΪ4��5�������£�Cu2+������ˮ�⣬��Fe3+������ȫˮ�⡣ijѧ���õ�ⴿ����CuSO4��Һ�ķ����������ݵ缫������Cu��������n���Լ��缫�ϲ�������������V mL ��״�������ⶨCu�����ԭ���������������£�

�ش��������⣺
��1������CuO�������� ��
��2������������õIJ�����������ͼ��ʾ����A��B�ֱ���ֱ����Դ�� �� �����������������
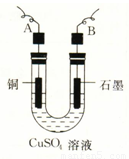
��3����ʼ����U�ι��п��Թ۲쵽�������У� ���������ӷ���ʽΪ ��
��4������ʵ������б�Ҫ���� ����д��ĸ����
��A���������ǰ�ĵ缫����������B�����缫�ں�ɳ���ǰ������������ˮ��ϴ����C�����µ���缫��������ͭ������ϴ����������D�������ɳ��صIJ����б��밴����ɡ��������ٺ�ɡ��ٳ��������У���E�����п������ڵ�����£���ɵ缫�����õ��º�ɵķ�����
��5��ͭ�����ԭ������Ϊ ���ô���m��V�ļ���ʽ��ʾ����
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ŨH2SO4�� |
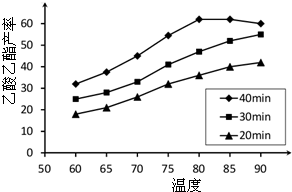
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
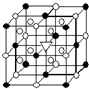 A����1����ͼ��ʾΪ����ʯ����ѧʽΪNa3AlF6���ľ�����ͼ�С�λ�ڴ������嶥������ģ���λ�ڴ��������12������е��8��С����������ģ���ͼ�С��е�һ�֣�ͼ�С�ֱ�ָ����������
A����1����ͼ��ʾΪ����ʯ����ѧʽΪNa3AlF6���ľ�����ͼ�С�λ�ڴ������嶥������ģ���λ�ڴ��������12������е��8��С����������ģ���ͼ�С��е�һ�֣�ͼ�С�ֱ�ָ����������| �۵�/K | �е�/K | ��״��ʱ��ˮ�е��ܽ�� | |
| H2S | 187 | 202 | 2.6 |
| H2O2 | 272 | 423 | ������Ȼ��� |
| 80m-135n |
| 18n |
| 80m-135n |
| 18n |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
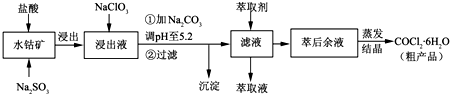
| ������ | Fe��OH��3 | Fe��OH��2 | Co��OH��2 | Al��OH��3 | Mn��OH��2 |
| ��ʼ���� | 2.7 | 7.6 | 7.6 | 4.0 | 7.7 |
| ��ȫ���� | 3.7 | 9.6 | 9.2 | 5.2 | 9.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ������ѧ����3��˫����ϰ��������ѧ�Ծ����������� ���ͣ������
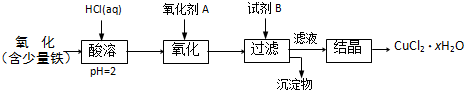
��20�֣��ۺ��Ȼ�����һ�����͡���Ч�������;�ˮ�����䵥����Һ̬�ļ�ʽ�Ȼ���[Al2��OH��nCl6-n]����ʵ�����������Һˮ���������Ʊ���ʽ�Ȼ��������Ʊ�ԭ��Ϊ�ֲ��㡢�۸����ĸ���������ѧ���Ϊ��Al2O3��25%��34%����SiO2��40%��50%����Fe2O3��0��5%��3��0%���Լ��������ʺ�ˮ�֡���֪�������ж��ֲ�ͬ�Ľṹ����ѧ����Ҳ�в��죬��һ�������¿��ת�����������е��������������ᡣ�Ʊ���ʽ�Ȼ�����ʵ���������£�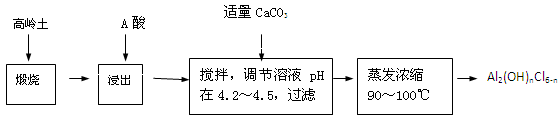
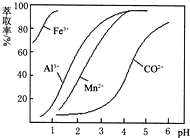
��֪��Fe3����Al3��������������ʽ��ȫ����ʱ����Һ��pH�ֱ�Ϊ3.2��5.2��
��������ͼ�ش��������⣺
��1�������ա���Ŀ����_______________________________________________��
��2���������������з�����Ӧ�����ӷ���ʽΪ_______________________________��
��3����������ѡ�õ���Ϊ_______��������������15����A����Ҫ200mL30����A�ᣨ�ܶ�ԼΪ1.15g/cm3����_______g����ˮ�������õ����������ձ�����������______________��
��4��Ϊ������Ľ����ʣ��ɲ�ȡ�Ĵ�ʩ�� _______________��Ҫ��д����������
��5����������ҺpH��4��2��4��5���Ĺ����У������ӱ�Ҫ���Լ������������ʵ����Ʒ��_________________��������Ũ�����豣���¶���90��100�棬�����¶ȵ�ʵ�鷽����___________ ______��
��6��ʵ�����Ʊ���ʽ�Ȼ�����Ӧ�Ļ�ѧ����ʽΪ_________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꽭��ʡ�����ڶ���ģ�⿼�Ի�ѧ�Ծ� ���ͣ������
��20�֣��ۺ��Ȼ�����һ�����͡���Ч�������;�ˮ�����䵥����Һ̬�ļ�ʽ�Ȼ���[Al2��OH��nCl6-n]����ʵ�����������Һˮ���������Ʊ���ʽ�Ȼ��������Ʊ�ԭ��Ϊ�ֲ��㡢�۸����ĸ��� ������ѧ���Ϊ��Al2O3��25%��34%����SiO2��40%��50%����Fe2O3��0��5%��3��0%���Լ��������ʺ�ˮ�֡���֪�������ж��ֲ�ͬ�Ľṹ����ѧ����Ҳ�в��죬��һ�������¿��ת�����������е��������������ᡣ�Ʊ���ʽ�Ȼ�����ʵ���������£�
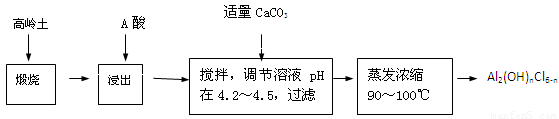
��֪��Fe3����Al3��������������ʽ��ȫ����ʱ����Һ��pH�ֱ�Ϊ3.2��5.2��
��������ͼ�ش��������⣺
��1�������ա���Ŀ����_______________________________________________��
��2���������������з�����Ӧ�����ӷ���ʽΪ_______________________________��
��3����������ѡ�õ���Ϊ_______��������������15����A����Ҫ200mL30����A�ᣨ�ܶ�ԼΪ1.15g/cm3����_______g����ˮ�������õ����������ձ�����������______________��
��4��Ϊ������Ľ����ʣ��ɲ�ȡ�Ĵ�ʩ�� _______________��Ҫ��д����������
��5����������ҺpH��4��2��4��5���Ĺ����У������ӱ�Ҫ���Լ������������ʵ����Ʒ��_________________��������Ũ�����豣���¶���90��100�棬�����¶ȵ�ʵ�鷽����___________ ______��
��6��ʵ�����Ʊ���ʽ�Ȼ�����Ӧ�Ļ�ѧ����ʽΪ_________________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com