
 ��
��
 ��
�� ���ĺ����Ͼ�������ϵ�д�
���ĺ����Ͼ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
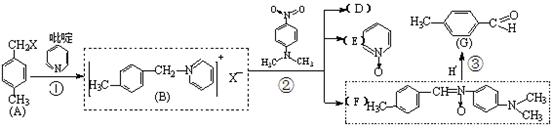
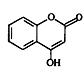
| A������������KMnO4��Һ����A��G |
| B��G���ʿ�������Ϊ3-������ȩ |
| C��A��G�ĺϳ�·�߽�����Krohnke��Ӧ�ڶ��л�����ʱ���������������� |
| D��A��G���ʺ˴Ź������������4�ַ� |
�鿴�𰸺ͽ���>>
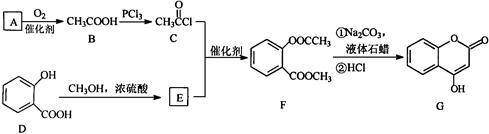
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
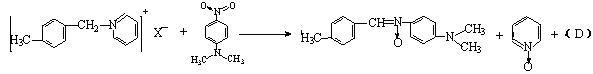
 A�ɷ������б仯����B��D��E���ɷ���������Ӧ��G�ĺ˴Ź���������ֻ��һ�����շ塣
A�ɷ������б仯����B��D��E���ɷ���������Ӧ��G�ĺ˴Ź���������ֻ��һ�����շ塣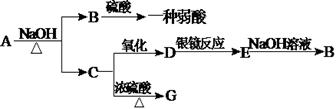
 �ƶ��������ʵĽṹ��ʽ��
�ƶ��������ʵĽṹ��ʽ���鿴�𰸺ͽ���>>
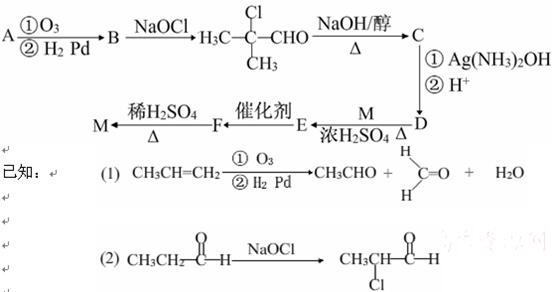
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
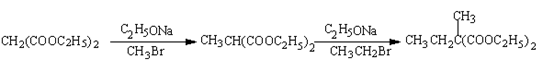
 �л���A��һ�������¿��Է�������ͼ��ʾ��ת������������
�л���A��һ�������¿��Է�������ͼ��ʾ��ת������������ ��ˮ��ʡ�ԣ�������������ʵĽṹ������Ϊ��A����������������Ʒ�Ӧ����������B����̼ԭ������A��ͬ����ֻ����һ�ֹ���
��ˮ��ʡ�ԣ�������������ʵĽṹ������Ϊ��A����������������Ʒ�Ӧ����������B����̼ԭ������A��ͬ����ֻ����һ�ֹ��� �ţ�C�ܷ���������Ӧ��1molD������̼��������Һ��Ӧ�ɲ���������̼2mol��
�ţ�C�ܷ���������Ӧ��1molD������̼��������Һ��Ӧ�ɲ���������̼2mol��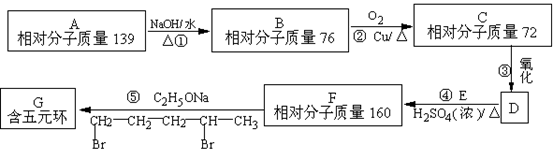
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
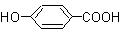
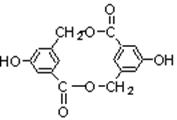
�鿴�𰸺ͽ���>>
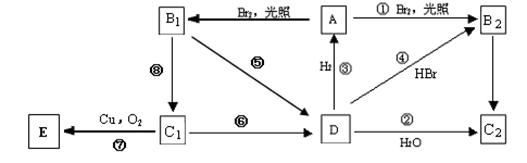
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A����Ӧ�����ڼӳɷ�Ӧ |
| B��X��Y��W��Z����ʹ���Ը��������ɫ |
| C��X��Y��W��Z������NaOH��Һ��Ӧ |
| D��1 mol��X��W�����ܹ�����3 mol Br2 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com