
��12�֣������ᴿ���о���
���й����ϡ�
| ��ѧʽ | CaCO3 | CaSO3 | CaC2O4 | Mg(OH)2 |
| Ksp | 4.96��10��9 | 4.96��10��9 | 2.34��10��9 | 5.61��10��12 |
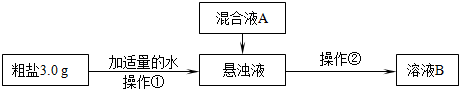
ij�о���ѧϰС��Դ��ε��ᴿ�ͼ�������о��������һЩ�µķ�������֪�ô�����Ʒ����Ҫ���в��������ʡ�Mg2+��Ca2+�ȣ�����SO42���Ĵ��ڣ�����С������������£�
 ����Ƴ��ӹ��̡�
����Ƴ��ӹ��̡�
��1������������Ҫʹ�õIJ��������� �� �������ڵ�����Ϊ ��
��2�����ҺA����Ҫ�ɷ��� �����ѧʽ��
�������������
��3��Ϊ������ҺB��Mg2+��Ca2+�Ƿ������ͨ���ֱ�ȡ������ҺB����֧�Թ��У���������ʵ�飺
����һ������Mg2+�Ƿ������������һ֧�Թ��м��� ��Һ���ѧʽ�������Ƿ��г������ɡ�
�����������Ca2+�Ƿ����������һ֧�Թ��м���ij��Һ�����Ƿ��г������ɡ�Ч����õ��� ������ĸ����A��Na2CO3 B��Na2SO3 C��Na2C2O4
����ȡ����ʳ�Ρ�
��4������ҺB���Ȳ����ϵμ�6 mol��L��1��������Һ��ͬʱ��pH��ֽ�����Һ��ֱ��pH=2ʱֹͣ�����ᣬ�õ���ҺC���ò�����Ŀ���� ��
��5������ҺC���� �����������ƣ��У������������ò��������Ͻ��裬ֱ�� ʱ��������ֹͣ���ȡ�
���������ۡ�
��6���ڳ��ӹ����У����������Һ�мӻ��ҺA���ò����п�����ҺpH=12��ȷ��Mg2+�����������ṩ�����ݼ��㣬��ҺB��Mg2+���ʵ���Ũ�Ƚ��������� ���¡�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ��ѧʽ | CaCO3 | CaSO3 | CaC2O4 | Mg��OH��2 |
| Ksp | 4.96��10-9 | 4.96��10-9 | 2.34��10-9 | 5.61��10-12 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��ѧʽ | CaCO3 | CaSO3 | CaC2O4 | Mg��OH��2 |
| Ksp | 4.96��10-9 | 4.96��10-9 | 2.34��10-9 | 5.61��10-12 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�����ᴿ���о���
![]() ���й����ϡ�
���й����ϡ�
��ѧʽ | CaCO3 | CaSO3 | CaC2O4 | Mg(OH)2 |
Ksp | 4.96��10�D9 | 4.96��10�D9 | 2.34��10�D9 | 5.61��10�D12 |
ij�о���ѧϰС��Դ��ε��ᴿ�ͼ�������о��������һЩ�µķ�������֪�ô�����Ʒ����Ҫ���в��������ʡ�Mg2+��Ca2+��(����SO42�D�Ĵ���),��С������������£�
![]()

����Ƴ��ӹ��̡�
![]()
![]()
(1)����������Ҫʹ�õIJ��������� �� �������ڵ�����Ϊ �����ڲ����ڽ���������ҺB���л��ǣ�Ӧ��ȡ�Ĵ�ʩ��_ _____________________________________________________��
(2)���ҺA����Ҫ�ɷ���____________________��(�ѧʽ)
�������������
(3)Ϊ������ҺB��Mg2+��Ca2+�Ƿ������ͨ���ֱ�ȡ������ҺB����֧�Թ��У���������ʵ�飺
����һ������Mg2+�Ƿ������������һ֧�Թ��м��� ��Һ(�ѧʽ)�����Ƿ��г������ɡ�
�����������Ca2+�Ƿ����������һ֧�Թ��м���ij��Һ�����Ƿ��г������ɡ�Ч����õ��� (����ĸ)��
A��Na2CO3 B��Na2SO
����ȡ����ʳ�Ρ�
(4)����ҺB���Ȳ����ϵμ�6 mol?L�D1��������Һ��ͬʱ��pH��ֽ�����Һ��ֱ��pH=5ʱֹͣ�����ᣬ�õ���ҺC���ò�����Ŀ���� ��
(5)����ҺC���� (����������)�У������������ò��������Ͻ��裬ֱ�� ![]() ___________ _____________ʱ(������)��ֹͣ���ȡ�
___________ _____________ʱ(������)��ֹͣ���ȡ�
���������ۡ�
(6)�ڳ��ӹ����У����������Һ�мӻ��ҺAʱ��Ҫ���ȣ�Ŀ���� ���ò����п�����ҺpH=12��ȷ��Mg2+�����������ṩ�����ݼ��㣬��ҺB��Mg2+���ʵ���Ũ�Ƚ���������______________���¡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009�긣��ʡ�������������и߿���ѧģ�����������һ���������棩 ���ͣ������
| ��ѧʽ | CaCO3 | CaSO3 | CaC2O4 | Mg��OH��2 |
| Ksp | 4.96×10-9 | 4.96×10-9 | 2.34×10-9 | 5.61×10-12 |

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com