


 ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д� A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д� ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| �ζ����� | ������Һ ���(mL) | ������� | |
| �ζ�ǰ�Ŀ̶� (mL) | �ζ���Ŀ̶� (mL) | ||
| ��һ�� | 10.00 | 0.40 | 20.50 |
| �ڶ��� | 10.00 | 4.10 | 24.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��2��10-12mol/L | B��1/2��10-9+10-12��mol/L |
| C����10-9+10-12��mol/L | D��1/2��10-5+10-2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��H2��ֵΪ142.9 kJ��g-1��������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ�� 2H2(g)+ O2(g)= 2H2O(l)��H=-285.8kJ��mol-1 |
| B��Al3����Cu2����SO42����F��������Һ�д������� |
| C������������Һ�ڿ����з���������Ӧ��4 Fe2���� O2��4H+�� 4 Fe3+��2H2O |
| D����֪KSP(Ag2SO4)= 1.4��10��5������100mL0.01mol��L��1��Na2SO4��Һ�м���1 mL0.01mol��L��1��AgNO3��Һ���а�ɫ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����һԪ����Һ��pH=11 |
| B������Һ����ˮ�������c��OH-��=1.0��10-11mol��L-1 |
| C������Һ��ˮ�����ӻ�����KWΪ1.0��10-14mol��L-2 |
| D����pH=1��������ҺV1L��V2L0.1mol��L-1��AOH��Һ��ϣ��������ҺpH=7����V1=V2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

 �������20.00mL 1.000mol
�������20.00mL 1.000mol
 ��ˮ�У���ҺpH���¶��������������仯��������ͼ��ʾ�������й�˵����ȷ����
��ˮ�У���ҺpH���¶��������������仯��������ͼ��ʾ�������й�˵����ȷ���� 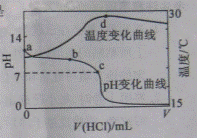
A��a����ˮ������� |
B��b�㣺 |
C��c�㣺 |
D��d��������¶����½�����Ҫԭ���� �������� �������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Q1 = Q2 = Q3 | B��2Q3 = Q1 < Q2 | C��Q3 < Q2 < Q1 | D��Q1 < Q2 < 3Q3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���¶ȵ����ߣ���ˮ��pH���� | B����ˮϡ�ͣ�̼������Һ��c(H��)��С |
| C���¶ȵ����ߣ�������Һ��c(H��)�������� | |
| D��pH = 5������ϡ��1000������Һ��pH��Ϊ8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ����ǰ��<����
����ǰ��<�����鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com