
��14�֣���֪�������������������ԭ��Ӧ��ʱ��һ������Ũ��Խϡ����Ӧ�Ļ�ԭ�����е��Ļ��ϼ�Խ�͡�����һ�������������Ͻ���һ����ϡHNO3��ַ�Ӧ����Ӧ���������κ�����ų����ڷ�Ӧ���������Һ�У���μ���4 mol/L NaOH��Һ������NaOH��Һ�������V����������������ʵ�����n����ϵ��ͼ��ʾ����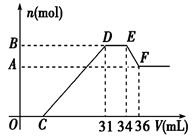
��1����д��DE�Ρ�EF����������Ӧ�����ӷ� ��ʽ��
��ʽ��
DE��
EF��
��2���Ͻ����������ʵ���Ϊ mol
��3���Ͻ��н��������ʵ�����Ϊ mol
��4�����C���ֵΪ ml
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣���֪�������������������ԭ��Ӧ��ʱ��һ������Ũ��Խϡ����Ӧ�Ļ�ԭ�����е��Ļ��ϼ�Խ�͡�����һ�������������Ͻ���һ����ϡHNO3��ַ�Ӧ����Ӧ���������κ�����ų����ڷ�Ӧ���������Һ�У���μ���4 mol/L NaOH��Һ������NaOH��Һ�������V����������������ʵ�����n����ϵ��ͼ��ʾ����
��1����д��DE�Ρ�EF����������Ӧ�����ӷ���ʽ��
DE��
EF��
��2���Ͻ����������ʵ���Ϊ mol
��3���Ͻ��н��������ʵ�����Ϊ mol
��4�����C���ֵΪ ml
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ������ѧ�ڵڶ��ζο���ѧ���� ���ͣ������
��14�֣���֪�������������������ԭ��Ӧ��ʱ��һ������Ũ��Խϡ����Ӧ�Ļ�ԭ�����е��Ļ��ϼ�Խ�͡�����һ�������������Ͻ���һ����ϡHNO3��ַ�Ӧ����Ӧ���������κ�����ų����ڷ�Ӧ���������Һ�У���μ���4 mol/L NaOH��Һ������NaOH��Һ�������V����������������ʵ�����n����ϵ��ͼ��ʾ����

��1����д��DE�Ρ�EF����������Ӧ�����ӷ���ʽ��
DE��
EF��
��2���Ͻ����������ʵ���Ϊ mol
��3���Ͻ��н��������ʵ�����Ϊ mol
��4�����C���ֵΪ ml
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�������������������ԭ��Ӧ��ʱ��һ������Ũ��Խϡ����Ӧ�Ļ�ԭ�����е��Ļ��ϼ�Խ�͡�����һ�������������Ͻ���һ����ϡHNO3��ַ�Ӧ����Ӧ���������κ�����ų����ڷ�Ӧ���������Һ�У���μ���4 mol/L NaOH��Һ������NaOH��Һ�������V����������������ʵ�����n����ϵ��ͼ��ʾ����

��1����д��DE�Ρ�EF����������Ӧ�����ӷ���ʽ��
DE��
EF��
��2���Ͻ����������ʵ���Ϊ mol
��3���Ͻ��н��������ʵ�����Ϊ mol
��4�����C���ֵΪ ml
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�������������������ԭ��Ӧ��ʱ��һ������Ũ��Խϡ����Ӧ�Ļ�ԭ�����е��Ļ��ϼ�Խ�͡�����һ�������������Ͻ���һ����ϡHNO3��ַ�Ӧ����Ӧ���������κ�����ų����ڷ�Ӧ���������Һ�У���μ���4 mol/L NaOH��Һ������NaOH��Һ�����(V)��������������ʵ���(n)��ϵ��ͼ��ʾ����
��1����д��DE�Ρ�EF����������Ӧ�����ӷ���ʽ��
DE������������������������������ ������
EF������������������������������
��2���Ͻ����������ʵ���Ϊ�������������� mol
��3���Ͻ��н��������ʵ�����Ϊ������������ mol
��4�����C���ֵΪ���������� ml

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com