
��14�֣�ij�����A������KAl(SO4)2��Al2O3��Fe2O3����һ�������¿�ʵ����ͼ��ʾ������֮��ı仯��

�ݴ˻ش��������⣺
��1��I��II��III��IV�IJ��ж�����Һ�ͳ����ķ����ȡ�ķ����� ��
��2������������ͼ��Ӧ��ϵ��д������B��C��D��E�������ʵĻ�ѧʽ
����B ������C ������D ��
��3��д���١��ڡ���������Ӧ����ʽ���������ӷ�Ӧ��д���ӷ���ʽ��
�� ��
�� ��
�� ��
��ÿ��2�֣���14�֣���1�����ˣ�2��Al2O3 Al2O3��Fe2O3 Fe2O3
��3����Al2O3��2NaOH��2NaAlO2��H2O ��Al3+��3NH3��H2O��Al(OH) 3����3NH4+
��2Al(OH) 3 Al2O3��3H2O
Al2O3��3H2O
��������
���������������������ˮ�ģ�������������������������ˮ�����Գ���C�����������������Ļ�������Һ����������Һ�����������İ�ˮ��Ӧ��������������ɫ����������李�����أ�����ҺE������李�����ؼ������İ�ˮ�������������ȷֽ�����������������B����������������������������Һ����Ӧ������D����������������Һ��ƫ�����ƺ������������ơ�Ȼ��������������ᣬ�Ϳ�����������������ɫ������
���㣺�������ʵķ�����ᴿ
�������������л��е����ʳ�ȥ����ô�������ᴿ���������һ��IJ�ͬ���ʱ˴˷ֿ����õ���Ӧ��ֵĸ�������з��롣�ڽ�����ʷ����ᴿ����ʱ,ѡ���Լ���ʵ���������Ӧ��ѭ����ԭ��: 1.���������µ����ʣ�ˮ���⣩���������ᴿ�������Ӧ�Ǵ����������Һ�����������������ʻ������У�2.�����ᴿ�������״̬���䣻3.ʵ����̺Ͳ������������У���ѡ������ᴿ����Ӧ��ѭ��������ѧ���ȼ��ӵ�ԭ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�ݴ��жϣ�
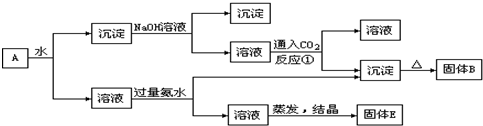
��1������B�������ʵĻ�ѧʽΪ_______________��
��2������E�������ʵĻ�ѧʽΪ_______________________��
��3����Ӧ�ٵ����ӷ���ʽΪ_______________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com