
œ¬ΆΦ « Β―ι “÷Τ±Η¬»Τχ≤ΔΫχ––“ΜœΒΝ–œύΙΊ Β―ιΒΡΉΑ÷Ο(Φ–≥÷…η±Η“―¬‘)ΓΘ
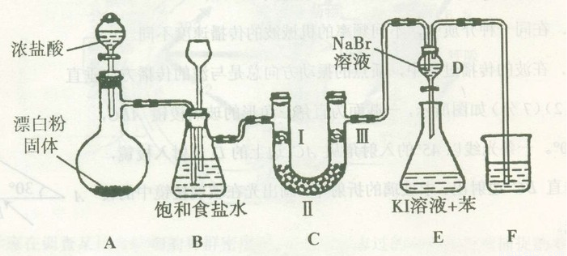
Θ®1Θ©ΉΑ÷ΟA «¬»ΤχΒΡΖΔ…ζΉΑ÷ΟΘ§«κ–¥≥ωœύ”ΠΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΘΚ?????????? ΓΘ
Θ®2Θ©ΉΑ÷ΟB÷–±ΞΚΆ ≥―ΈΥ°ΒΡΉς”Ο «??????????? ΘΜΆ§ ±ΉΑ÷ΟB“≤ «Α≤»ΪΤΩΘ§Φύ≤β Β―ιΫχ–– ±C÷– «ΖώΖΔ…ζΕ¬»ϊΘ§«κ–¥≥ωΖΔ…ζΕ¬»ϊ ±B÷–ΒΡœ÷œσΘΚ??????????????? ΓΘ
Θ®3Θ©ΉΑ÷ΟCΒΡ Β―ιΡΩΒΡ «―ι÷Λ¬»Τχ «ΖώΨΏ”–Τ·ΑΉ–‘Θ§ΈΣ¥ΥC÷–IΓΔIIΓΔIII¥Π“ά¥ΈΖ≈»κΈο÷ ΒΡΉιΚœ”Π «??????? (ΧνΉ÷ΡΗ±ύΚ≈)ΓΘ
±ύΚ≈ | I | II | III |
a | Η…‘οΒΡ”–…Ϊ≤ΦΧθ | Φν ·Μ“ | Σ»σΒΡ”–…Ϊ≤ΦΧθ |
b | Η…‘οΒΡ”–…Ϊ≤ΦΧθ | ΈόΥ°ΝρΥαΆ≠ | Σ»σΒΡ”–…Ϊ≤ΦΧθ |
c | Σ»σΒΡ”–…Ϊ≤ΦΧθ | ≈®ΝρΥα | Η…‘οΒΡ”–…Ϊ≤ΦΧθ |
d | Σ»σΒΡ”–…Ϊ≤ΦΧθ | ΈόΥ°¬»Μ·ΗΤ | Η…‘οΒΡ”–…Ϊ≤ΦΧθ |
Θ®4Θ©…ηΦΤΉΑ÷ΟDΓΔEΒΡΡΩΒΡ «±»Ϋœ¬»ΓΔδεΓΔΒβΒΞ÷ ΒΡ―θΜ·–‘ΓΘΖ¥”Π“ΜΕΈ ±ΦδΚσΘ§¥ρΩΣΜν»ϊΘ§ΫΪΉΑ÷ΟD÷–…ΌΝΩ»ή“ΚΦ”»κΉΑ÷ΟE÷–Θ§’ώΒ¥Θ§Ιέ≤λΒΫΒΡœ÷œσ « ????????????????????????? Θ§ΗΟœ÷œσ?????????? (ΧνΓΑΡήΓ±ΜρΓΑ≤ΜΡήΓ±)ΥΒΟςδεΒΞ÷ ΒΡ―θΜ·–‘«Ω”ΎΒβΘ§‘≠“ρ «???????????? ΓΘ
Θ®5Θ©ΉΑ÷ΟFΒΡΉς”Ο «?????????????????? Θ§Τδ…’±≠÷–ΒΡ»ή“Κ≤ΜΡή―Γ”Οœ¬Ν–÷–ΒΡ ???? (ΧνΉ÷ΡΗ±ύΚ≈)ΓΘ
aΘ°±ΞΚΆNaOH»ή“Κ????? bΘ°±ΞΚΆCa(OH)2»ή“Κ
cΘ°±ΞΚΆNa2SO3»ή“Κ???? dΘ°±ΞΚΆNa2CO3»ή“Κ
Θ®1Θ©Ca(ClO) 2ΘΪ4HCl(≈®)=CaCl2ΘΪ2Cl2ΓϋΘΪ2H2O
Θ®2Θ©≥ΐ»ΞCl2÷–ΒΡHClΓΓ????? B÷–≥ΛΨ±¬©ΕΖ÷–“ΚΟφ…œ…ΐΘ§–Έ≥…Υ°÷υ
Θ®3Θ©d
Θ®4Θ©E÷–»ή“ΚΖ÷ΈΣΝΫ≤ψΘ§…œ≤ψ(±Ϋ≤ψ)ΈΣΉœΚλ…Ϊ? ?? ≤ΜΡήΓΓ???? ΙΐΝΩΒΡCl2“≤Ω…ΫΪIΘ≠―θΜ·ΈΣI2
Θ®5Θ©Έϋ ’”ύ¬»Θ§Ζά÷ΙΈέ»Ψ¥σΤχ???? b
ΓΨΫβΈωΓΩ
‘ΧβΖ÷ΈωΘΚΘ®1Θ©Τ·ΑΉΖέ÷–ΒΡCa(ClO)2”κ≈®―ΈΥαΖ¥”Π…ζ≥…Cl2Θ§Μ·―ßΖΫ≥Χ ΫΈΣΘΚCa(ClO) 2ΘΪ4HCl(≈®)==CaCl2ΘΪ2Cl2ΓϋΘΪ2H2O
Θ®2Θ©”Ο≈®―ΈΥα÷Τ»ΓΒΡCl2÷–Κ§”–HClΘ§Υυ“‘ΉΑ÷ΟB÷–±ΞΚΆ ≥―ΈΥ°ΒΡΉς”Ο «≥ΐ»ΞCl2÷–ΒΡHClΘΜ»γΙϊC÷–ΖΔ…ζΕ¬»ϊΘ§B÷–―Ι«Ω‘ω¥σΘ§‘ρB÷–≥ΛΨ±¬©ΕΖ÷–“ΚΟφ…œ…ΐΘ§–Έ≥…Υ°÷υΓΘ
Θ®3Θ©ΈΣΝΥ―ι÷Λ¬»Τχ «ΖώΨΏ”–Τ·ΑΉ–‘Θ§I÷–Φ”»κ Σ»σΒΡ”–…Ϊ≤ΦΧθΘ§IIΈΣU–ΆΙήΘ§Ω…Φ”»κΙΧΧεΗ…‘οΦΝΘ§ΒΟΒΫΗ…‘οΒΡCl2Θ§III÷–Φ”»κΗ…‘οΒΡ”–…Ϊ≤ΦΧθΘ§Φ¥Ω…÷ΛΟςCl2 «ΖώΨΏ”–Τ·ΑΉ–‘Θ§Ι dœν’ΐ»ΖΓΘ
Θ®4Θ©ΉΑ÷ΟD÷– ‘ΦΝΈΣδεΥ°Θ§Φ”»κΒΫE÷–Θ§Br2”κI?Ζ¥”Π…ζ≥…I2Θ§Υυ“‘ Β―ιœ÷œσΈΣΘΚE÷–»ή“ΚΖ÷ΈΣΝΫ≤ψΘ§…œ≤ψ(±Ϋ≤ψ)ΈΣΉœΚλ…ΪΘΜCl2Ά®»κD”κNaBrΖ¥”Π…ζ≥…Br2Θ§Ω…ΡήΜα”–Cl2 Θ”ύΘ§Υυ“‘Α―D÷–»ή“ΚΦ”»κΒΫE÷–Θ§“≤”–Ω…Ρή «Cl2ΫΪI?―θΜ·ΈΣI2Θ§“ρ¥Υ≤ΜΡήΥΒΟςδεΒΞ÷ ΒΡ―θΜ·–‘«Ω”ΎΒβΓΘ
Θ®5Θ©Cl2”–ΕΨΘ§–η“ΣΫχ––Έ≤Τχ¥ΠάμΘ§Υυ“‘ΉΑ÷ΟFΒΡΉς”Ο «Έϋ ’”ύ¬»Θ§Ζά÷ΙΈέ»Ψ¥σΤχΘΜAΓΔCl2Ρή”κNaOH»ή“ΚΖ¥”ΠΘ§±ΜΈϋ ’Θ§’ΐ»ΖΘΜbΓΔ“ρΈΣCa(OH)2ΈΔ»ή”ΎΥ°Θ§±ΞΚΆCa(OH)2»ή“Κ≈®Ε»–ΓΘ§≤ΜΡή≥δΖ÷Έϋ ’Cl2Θ§¥μΈσΘΜCΓΔCl2ΨΏ”–Ϋœ«Ω―θΜ·–‘Θ§Ρή―θΜ·Na2SO3Θ§±ΜΈϋ ’Θ§’ΐ»ΖΘΜDΓΔCl2Ρή”κNa2CO3»ή“ΚΖ¥”ΠΘ§…ζ≥…NaClΓΔNaClOΓΔNaHCO3Θ§±ΜΈϋ ’Θ§’ΐ»ΖΓΘ
ΩΦΒψΘΚ±ΨΧβΩΦ≤ιΜυ±Ψ≤ΌΉςΓΔ Β―ιΖΫΑΗΒΡ…ηΦΤ”κΖ÷ΈωΓΔΜ·―ßΖΫ≥Χ ΫΒΡ ι–¥ΓΘ


 »Ϊ”≈≥ε¥Χ100Ζ÷œΒΝ–¥πΑΗ
»Ϊ”≈≥ε¥Χ100Ζ÷œΒΝ–¥πΑΗ ”Δ≤≈ΒψΫρœΒΝ–¥πΑΗ
”Δ≤≈ΒψΫρœΒΝ–¥πΑΗ
| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ‘ΡΕΝάμΫβ
| a | b | c | d | |
| Δώ | Η…‘οΒΡ”–…Ϊ≤ΦΧθ | Η…‘οΒΡ”–…Ϊ≤ΦΧθ | Σ»σΒΡ”–…Ϊ≤ΦΧθ | Σ»σΒΡ”–…Ϊ≤ΦΧθ |
| Δρ | Φν ·Μ“ | ΙηΫΚ | ≈®ΝρΥα | ΈόΥ°¬»Μ·ΗΤ |
| Δσ | Σ»σΒΡ”–…Ϊ≤ΦΧθ | Σ»σΒΡ”–…Ϊ≤ΦΧθ | Η…‘οΒΡ”–…Ϊ≤ΦΧθ | Η…‘οΒΡ”–…Ϊ≤ΦΧθ |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2013ΫλΧλΫρ ––¬ΜΣ÷–―ßΗΏ»ΐΒΎ»ΐ¥Έ‘¬ΩΦΜ·―ß ‘ΨμΘ®¥χΫβΈωΘ© Χβ–ΆΘΚ Β―ιΧβ
œ¬ΆΦ « Β―ι “÷Τ±Η¬»Τχ≤ΔΫχ––“ΜœΒΝ–œύΙΊ Β―ιΒΡΉΑ÷ΟΘ®Φ–≥÷…η±Η“―¬‘Θ©ΓΘ
Θ®1Θ©÷Τ±Η¬»Τχ―Γ”ΟΒΡ“©ΤΖΈΣΘΚΤ·ΑΉΖέΙΧΧεΚΆ≈®―ΈΥαΘ§œύΙΊΒΡΜ·―ßΖ¥”ΠΖΫ≥Χ ΫΈΣΘΚ___________________________________ΓΘ
Θ®2Θ©ΉΑ÷ΟB÷–±ΞΚΆ ≥―ΈΥ°ΒΡΉς”Ο «_______ΘΜΆ§ ±ΉΑ÷ΟB“ύ «Α≤»ΪΤΩΘ§Φύ≤β Β―ιΫχ–– ±C÷– «ΖώΖΔ…ζΕ¬»ϊΘ§«κ–¥≥ωΖΔ…ζΕ¬»ϊ ±B÷–ΒΡœ÷œσ______________________________ΓΘ
Θ®3Θ©ΉΑ÷ΟCΒΡ Β―ιΡΩΒΡ «―ι÷Λ¬»Τχ «ΖώΨΏ”–Τ·ΑΉ–‘Θ§ΈΣ¥ΥC÷–ΔώΓΔΔρΓΔΔσ“ά¥ΈΖ≈»κ__________ΓΘ
| | a | b | c | d |
| Δώ | Η…‘οΒΡ”–…Ϊ≤ΦΧθ | Η…‘οΒΡ”–…Ϊ≤ΦΧθ | Σ»σΒΡ”–…Ϊ≤ΦΧθ | Σ»σΒΡ”–…Ϊ≤ΦΧθ |
| Δρ | Φν ·Μ“ | ΙηΫΚ | ≈®ΝρΥα | ΈόΥ°¬»Μ·ΗΤ |
| Δσ | Σ»σΒΡ”–…Ϊ≤ΦΧθ | Σ»σΒΡ”–…Ϊ≤ΦΧθ | Η…‘οΒΡ”–…Ϊ≤ΦΧθ | Η…‘οΒΡ”–…Ϊ≤ΦΧθ |
 »ή“ΚΘ§≈–ΕœΗΡ”ΟNaHSO
»ή“ΚΘ§≈–ΕœΗΡ”ΟNaHSO »ή“Κ «ΖώΩ…––__________Θ®ΧνΓΑ «Γ±ΜρΓΑΖώΓ±Θ©ΓΘ»γΙϊ¥πΓΑΖώΓ±Θ§”ΟάκΉ”ΖΫ≥Χ ΫΫβ Ά‘≠“ρ____________________Θ®¥πΓΑ «Γ±‘ρ≤Μ”ΟΫβ ΆΘ©ΓΘ
»ή“Κ «ΖώΩ…––__________Θ®ΧνΓΑ «Γ±ΜρΓΑΖώΓ±Θ©ΓΘ»γΙϊ¥πΓΑΖώΓ±Θ§”ΟάκΉ”ΖΫ≥Χ ΫΫβ Ά‘≠“ρ____________________Θ®¥πΓΑ «Γ±‘ρ≤Μ”ΟΫβ ΆΘ©ΓΘ≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2012ΫλΚ”±± ΓΚβΥ°÷–―ßΗΏ»ΐœ¬―ßΤΎΕΰΒςΩΦ ‘άμΉέ ‘ΨμΘ®¥χΫβΈωΘ© Χβ–ΆΘΚ Β―ιΧβ
œ¬ΆΦ « Β―ι “÷Τ±Η¬»Τχ≤ΔΫχ––“ΜœΒΝ–œύΙΊ Β―ιΒΡΉΑ÷ΟΘ®Φ–≥÷…η±Η“―¬‘Θ©ΓΘ
Δ≈÷Τ±Η¬»Τχ―Γ”ΟΒΡ“©ΤΖΈΣΘΚΤ·ΖέΨΪΙΧΧεΚΆ≈®―ΈΥαΘ§œύΙΊΒΡΜ·―ßΖ¥”ΠΖΫ≥Χ ΫΈΣΘΚ
__________________________________________________________________ΓΘ
ΔΤΉΑ÷ΟB÷–±ΞΚΆ ≥―ΈΥ°ΒΡΉς”Ο «_______________ΘΜΆ§ ±ΉΑ÷ΟB“ύ «Α≤»ΪΤΩΘ§Φύ≤β Β―ιΫχ–– ±C÷– «ΖώΖΔ…ζΕ¬»ϊΘ§«κ–¥≥ωΖΔ…ζΕ¬»ϊ ±B÷–ΒΡœ÷œσ______________________________ΓΘ
Δ«ΉΑ÷ΟCΒΡ Β―ιΡΩΒΡ «―ι÷Λ¬»Τχ «ΖώΨΏ”–Τ·ΑΉ–‘Θ§ΈΣ¥ΥC÷–IΓΔIIΓΔIII“ά¥ΈΖ≈»κ_______ΓΘ
| | a | b | c | d |
| I | Η…‘οΒΡ”–…Ϊ≤ΦΧθ | Η…‘οΒΡ”–…Ϊ≤ΦΧθ | Σ»σΒΡ”–…Ϊ≤ΦΧθ | Σ»σΒΡ”–…Ϊ≤ΦΧθ |
| II | Φν ·Μ“ | ΙηΫΚ | ≈®ΝρΥα | ΈόΥ°¬»Μ·ΗΤ |
| III | Σ»σΒΡ”–…Ϊ≤ΦΧθ | Σ»σΒΡ”–…Ϊ≤ΦΧθ | Η…‘οΒΡ”–…Ϊ≤ΦΧθ | Η…‘οΒΡ”–…Ϊ≤ΦΧθ |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2013-2014―ßΡξ…ΫΕΪ ΓΒ¬÷ί –ΗΏ»ΐ1‘¬‘¬ΩΦΜ·―ß ‘ΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ Β―ιΧβ
λœΛ Β―ι“«ΤςΘ§Ρή’ΐ»ΖΫχ–– Β―ι≤ΌΉς «ΉωΚΟΜ·―ß Β―ιΒΡ«ΑΧαΓΘ
Θ®1Θ©œ¬Ν–”–ΙΊ Β―ι≤ΌΉςΜρ Β―ι ¬ ΒΒΡ–π ωΘ§’ΐ»ΖΒΡ « (Χν–ρΚ≈)ΓΘ
AΘ° Β―ι “÷–≈®œθΥα”Π±Θ¥φ‘ΎΉΊ…ΪœΗΩΎΤΩ÷–Θ§≤ΔΧυ”–»γΆΦΥυ Ψ±ξ«©

BΘ°”Ο50mLΝΩΆ≤ΝΩ»Γ5Θ°6mL≈®ΝρΥα
CΘ°÷–ΚΆΒΈΕ® Β―ι ±Θ§ΉΕ–ΈΤΩœ¥Β”Η…ΨΜ≤Δ”Ο±ξΉΦ“Κ»σœ¥ΚσΘ§ΖΫΩ…ΉΔ»κ¥ΐ≤β“Κ
DΘ°”ΟΥΡ¬»Μ·ΧΦίΆ»ΓΒβΥ°÷–ΒΡΒβΘ§Ζ÷“Κ ±”–Μζ≤ψ¥”Ζ÷“Κ¬©ΕΖΒΡœ¬ΕΥΖ≈≥ω
EΘ°”ΟΙψΖΚpH ‘÷Ϋ≤βΒΟΡ≥»ή“ΚΒΡpHΈΣ4Θ°8
Θ®2Θ©œ¬ΆΦ « Β―ι “÷Τ±Η¬»Τχ≤ΔΧΫΨΩ¬»Τχ «ΖώΨΏ”–Τ·ΑΉ–‘ΒΡ Β―ιΉΑ÷Ο(Φ–≥÷ΦΑΦ”»»“«Τς“― Γ¬‘)ΓΘ

ΔΌAΉΑ÷Ο÷–Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ ΓΘ
ΔΎBΉΑ÷Ο÷–“«ΤςaΒΡΟϊ≥Τ « ΓΘ
ΔέBΉΑ÷ΟΒΡΉς”Ο «≥ΐ»ΞΤχΧε÷–Μλ”–ΒΡHClΘ§ΦφΤπΑ≤»ΪΤΩΒΡΉς”ΟΘ§Β±“«Τςa÷–“ΚΟφ≤Μ
Εœ…œ…ΐ ±Θ§ΥΒΟς Θ§¥Υ ±”ΠΆΘ÷Ι Β―ιΓΘ
Δή Β―ι÷–Ιέ≤λΒΫ Θ§ΥΒΟςΗ…‘כּΤχΈόΤ·ΑΉ–‘ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2012-2013―ßΡξΚΰ±± ΓΨΘΟ≈ –ΗΏ»ΐ10‘¬‘¬ΩΦΜ·―ß ‘ΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚΦΤΥψΧβ
Θ®10Ζ÷Θ©œ¬ΆΦ « Β―ι “÷Τ±Η¬»Τχ≤ΔΫχ––“ΜœΒΝ–œύΙΊ Β―ιΒΡΉΑ÷ΟΘ®Φ–≥÷…η±Η“―¬‘Θ©ΓΘ

Θ®1Θ©÷Τ±Η¬»Τχ―Γ”ΟΒΡ“©ΤΖΈΣΘΚΤ·ΖέΨΪΙΧΧεΚΆ≈®―ΈΥαΘ§œύΙΊΒΡΜ·―ßΖ¥”ΠΖΫ≥Χ ΫΈΣΘΚ
ΓΘ
Θ®2Θ©ΉΑ÷ΟB÷–±ΞΚΆ ≥―ΈΥ°ΒΡΉς”Ο « ΘΜΆ§ ±ΉΑ÷ΟB“ύ «Α≤»ΪΤΩΘ§Φύ≤β Β―ιΫχ–– ±C÷– «ΖώΖΔ…ζΕ¬»ϊΘ§«κ–¥≥ωΖΔ…ζΕ¬»ϊ ±B÷–ΒΡœ÷œσ ΓΘ
Θ®3Θ©ΉΑ÷ΟCΒΡ Β―ιΡΩΒΡ «―ι÷Λ¬»Τχ «ΖώΨΏ”–Τ·ΑΉ–‘Θ§ΈΣ¥ΥC÷–IΓΔIIΓΔIII“ά¥ΈΖ≈»κ ΓΘ
|
|
a |
b |
c |
d |
|
I |
Η…‘οΒΡ”–…Ϊ≤ΦΧθ |
Η…‘οΒΡ”–…Ϊ≤ΦΧθ |
Σ»σΒΡ”–…Ϊ≤ΦΧθ |
Σ»σΒΡ”–…Ϊ≤ΦΧθ |
|
II |
Φν ·Μ“ |
ΙηΫΚ |
≈®ΝρΥα |
ΈόΥ°¬»Μ·ΗΤ |
|
III |
Σ»σΒΡ”–…Ϊ≤ΦΧθ |
Σ»σΒΡ”–…Ϊ≤ΦΧθ |
Η…‘οΒΡ”–…Ϊ≤ΦΧθ |
Η…‘οΒΡ”–…Ϊ≤ΦΧθ |
Θ®4Θ©…ηΦΤΉΑ÷ΟDΓΔEΒΡΡΩΒΡ «±»Ϋœ¬»ΓΔδεΓΔΒβΒΡΖ«Ϋπ τ–‘ΓΘΒ±œρD÷–ΜΚΜΚΆ®»κΉψΝΩ¬»Τχ ±Θ§Ω…“‘Ω¥ΒΫΈό…Ϊ»ή“Κ÷πΫΞ±δΈΣ…νΉΊΚλ…ΪΘ§ΥΒΟς¬»ΒΡΖ«Ϋπ τ–‘¥σ”ΎδεΓΘ¥ρΩΣΜν»ϊΘ§ΫΪΉΑ÷ΟD÷–…ΌΝΩ»ή“ΚΦ”»κΉΑ÷ΟE÷–Θ§’ώΒ¥ΓΘΙέ≤λΒΫΒΡœ÷œσ « ΓΘΗΟœ÷œσ
Θ®ΧνΓΑΡήΓ±ΜρΓΑ≤ΜΡήΓ±Θ©ΥΒΟςδεΒΡΖ«Ϋπ τ–‘«Ω”ΎΒβΘ§‘≠“ρ « ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΙζΦ ―ß–Θ”≈―Γ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com