
��֪A��BΪ���嵥�ʣ�����һ��Ϊ����ɫ��DΪ�����������ʣ�CΪ��������Ǽ�������ת����ϵ��

(1)д���������ʵĻ�ѧʽ��A________��C________��
(2)д��C��ˮ��Һ��D��Ӧ�����ӷ�Ӧ����ʽ________��
(3)F��D��Ӧ�����ӷ�Ӧ����ʽ________��
(4)����E��Һ�е������ӽ��м��飺
����һ����NaOH��Һ������E��Һ�������ӣ�����ȡ________�����Թ��У����ý�ͷ�ι�ȡ________�����Թ��У�����Ϊ�Ȳ�����ɫ���������ɻ���ɫ������ɺ��ɫ���۽��ۣ�E��Һ�к���ij��������
����������������ա�����һ������KSCN��Һ������E��Һ�е������ӣ�
��____________�������Թ��е���KSCN��Һ���Σ�����������____________������Ϊ________________����________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
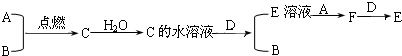
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
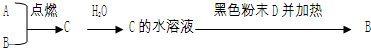
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��16�֣���֪A��BΪ���嵥�ʣ�����AΪ����ɫ���壬BΪ��ɫ���壻 CΪ�������ˮ��Һ�����ԣ�DΪ����ɫ�������ʣ�EΪdz��ɫ��Һ������֮��������ת����ϵ��
��1����д��A��B��D��E�Ļ�ѧʽ��
A_________��B_________��D____________��E________��
��2��д��A��B��Ӧ�Ļ�ѧ����ʽ�� ��
��3������E��Һ�м���NaOH��Һ����¶���ڿ�����һ��ʱ�䣬�ɹ۲쵽�������� ��
��4��E+A��Ӧ�����ӷ���ʽ�� ��
F+D��Ӧ�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��20111ѧ������ʡ����һ�и�һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��14�֣���֪A��BΪ���嵥�ʣ�����AΪ����ɫ���壬BΪ��ɫ���壻
CΪ�������ˮ��Һ��pHС��7��DΪ�������ʣ�����֮��������ת����ϵ��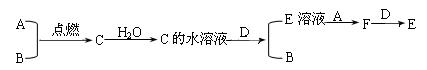
��1����д��A��B��C��D�Ļ�ѧʽ��
A_________��B_________��C___________��D_________��
��2��д��C��ˮ��Һ��D��Ӧ�����ӷ���ʽ��______________________;
E��Һ��A��Ӧ�����ӷ���ʽ��_______________________________��
F+D��Ӧ�����ӷ���ʽ��____________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com