
�Ҵ�����Ҫ���л�����ԭ�ϣ�������ϩ����ֱ��ˮ�Ϸ���������ˮ�Ϸ��������ش��������⣺
(1)���ˮ�Ϸ���ָ�Ƚ���ϩ��Ũ���ᷴӦ��������������(C2H5OSO3H)����ˮ�������Ҵ���д����Ӧ��Ӧ�Ļ�ѧ����ʽ��________________________________________��
(2)��֪��
�״�����ˮ��Ӧ
2CH3OH(g)===CH3OCH3(g)��H2O(g)
��H1����23.9 kJ��mol��1
�״���ϩ���ķ�Ӧ
2CH3OH(g)===C2H4(g)��2H2O(g)
��H2����29.1 kJ��mol��1
�Ҵ����칹����Ӧ��C2H5OH(g)===CH3OCH3(g)
��H3����50.7 kJ��mol��1
����ϩ����ֱ��ˮ�Ϸ�ӦC2H4(g)��H2O(g)===C2H5OH(g)�Ħ�H________kJ��mol��1������ˮ�Ϸ���ȣ�����ֱ��ˮ�Ϸ����ŵ���____________________________________��
(3)��ͼ��ʾΪ����ֱ��ˮ�Ϸ�����ϩ��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ[����n(H2O)��n(C2H4)��1��1]��
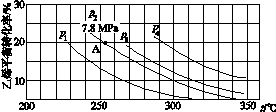
����ʽ������ϩˮ�����Ҵ���Ӧ��ͼ��A���ƽ�ⳣ��Kp��____________________(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ����ѹ�����ʵ�������)��
��ͼ��ѹǿ(p1��p2��p3��p4)�Ĵ�С˳��Ϊ____��������___________________��
������ֱ��ˮ�Ϸ������õĹ�������Ϊ������/������Ϊ��������Ӧ�¶�290 �桢ѹǿ6.9 MPa��n(H2O)��n(C2H4)��0.6��1����ϩ��ת����Ϊ5%����Ҫ��һ�������ϩת���ʣ����˿����ʵ��ı䷴Ӧ�¶Ⱥ�ѹǿ�⣬���ɲ�ȡ�Ĵ�ʩ��________________________________________________________________________��
________________________________________________________________________��
(1)C2H4��H2SO4�D��C2H5OSO3H��C2H5OSO3H��H2O�D��C2H5OH��H2SO4��(2)��45.5����ȾС����ʴ��С�ȡ�(3)�� ��
��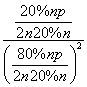 ��
��
 ��0.07(MPa)��1����p1<p2<p3<p4����Ӧ���������٣���ͬ�¶��£�ѹǿ������ϩת�������
��0.07(MPa)��1����p1<p2<p3<p4����Ӧ���������٣���ͬ�¶��£�ѹǿ������ϩת�������
�۽������Ҵ�Һ����ȥ������n(H2O)��n(C2H4)��
[����] (1)����������Ϣ��д������ϩ��Ũ������ˮ�Ϸ����Ҵ��ķ�ӦΪC2H4��H2SO4�D��C2H5OSO3H ��C2H5OSO3H��H2O�D��C2H5OH��H2SO4��(2)���ݸ�˹���ɢ٣��ڣ��۵ã�C2H4(g)��H2O(g)===C2H5OH(g)����H����45.5 kJ��mol��1�����ˮ�Ϸ����õ�Ũ�����ǿ��ʴ�����ʣ��������ֱ��ˮ�Ϸ�������ȾС����ʴ��С���ŵ㡣(3)������ʼʱC2H4��H2O(g)�����ʵ�����Ϊn������C2H4��ת����Ϊ20%����ƽ��ʱC2H4��H2O(g)��C2H5OH�����ʵ����ֱ�Ϊ80%n��80%n��20%n����Kp�� ��
��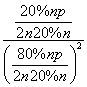 ��
�� ��0.07(MPa)��1��������ѹǿ��ƽ�⽫�����ƶ��������C2H4��ת���ʣ���ѹǿp1��p2��p3��p4����Ϊ��ʹƽ�������ƶ��������Խ��Ҵ�Һ����ʱ���룬������n (H2O)��n (C2H4) ֮�ȵȴ�ʩ��
��0.07(MPa)��1��������ѹǿ��ƽ�⽫�����ƶ��������C2H4��ת���ʣ���ѹǿp1��p2��p3��p4����Ϊ��ʹƽ�������ƶ��������Խ��Ҵ�Һ����ʱ���룬������n (H2O)��n (C2H4) ֮�ȵȴ�ʩ��


 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ�����Ʊ���������ʱ�����÷�����ȷ����(����)
A��������ʱ����Na2O2��H2O2����Ӧ���ѡ����ͬ�����巢��װ��
B��������ʱ���ñ���NaHCO3��Һ��Ũ���Ά������
C������ϩʱ������ˮ���������ſ������ռ�����
D���ƶ�������ʱ����ˮ��NaOH��Һ����β��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������̬���Ļ�����֪�����Ƕ���ʹ��ˮ��ɫ���ҷ�����̼ԭ������С��5,1����û��������ȫȼ�պɵõ�3.6���������̼��3���ˮ����(�����������ͬ��ͬѹ�²ⶨ)
(1)�����������������������________��
A���飬ϩ ���������� B��ϩ��ϩ
C��ϩ��Ȳ D��Ȳ��Ȳ
�����������жϵ�������________________________________
______________________________________________________________________________________________________________________��
(2)ͨ������ȷ�����������ķ���ʽ�Լ������ڻ�����е�����ȡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������AX3�͵���X2��һ�������·�Ӧ�����ɻ�����AX5���ش��������⣺
(1)��֪AX3���۵�ͷе�ֱ�Ϊ��93.6 ���76 �棬AX5���۵�Ϊ167 �档����ʱAX3������X2��Ӧ����1 mol AX5���ų�����123.8 kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ____________________________________________��
(2)��ӦAX3(g)��X2(g)AX5(g)���ݻ�Ϊ10 L���ܱ������н��С���ʼʱAX3��X2��Ϊ0.2 mol����Ӧ�ڲ�ͬ�����½��У���Ӧ��ϵ��ѹǿ��ʱ��ı仯��ͼ��ʾ��
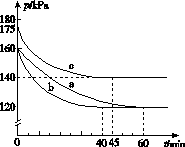
����ʽ����ʵ��a�ӷ�Ӧ��ʼ���ﵽƽ��ʱ�ķ�Ӧ����v(AX5)��______________________��
��ͼ��3��ʵ��ӷ�Ӧ��ʼ���ﵽƽ��ʱ�ķ�Ӧ����v(AX5)�ɴ�С�Ĵ���Ϊ____________(��ʵ�����)����ʵ��a��ȣ���������ı��ʵ���������ж������ǣ�b________________________________________________��c____________________________________________��
����p0��ʾ��ʼʱ��ѹǿ��p��ʾƽ��ʱ��ѹǿ������ʾAX3��ƽ��ת���ʣ������ı���ʽΪ______________��ʵ��a��c��ƽ��ת���ʣ���aΪ________����cΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ں����ܱ�������ͨ��X��������Ӧ��2X(g)Y(g)���¶�T1��T2��X�����ʵ���Ũ��c(X)��ʱ��t�仯��������ͼ��ʾ������������ȷ����(����)
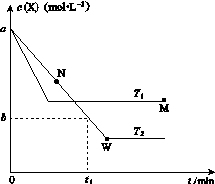
A���÷�Ӧ���е�M��ų����������ڽ��е�W��ų�������
B��T2�£���0��t1ʱ���ڣ�v(Y)��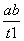 mol��L��1��min��1
mol��L��1��min��1
C��M�������Ӧ����v������N����淴Ӧ����v��
D��M��ʱ�ټ���һ����X��ƽ���X��ת���ʼ�С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������AX3�͵���X2��һ�������·�Ӧ�����ɻ�����AX5���ش��������⣺
(1)��֪AX3���۵�ͷе�ֱ�Ϊ��93.6 ���76 �棬AX5���۵�Ϊ167 �档����ʱAX3������X2��Ӧ����1 mol AX5���ų�����123.8 kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ____________________________________________��
(2)��ӦAX3(g)��X2(g)  AX5(g)���ݻ�Ϊ10 L���ܱ������н��С���ʼʱAX3��X2��Ϊ0.2 mol����Ӧ�ڲ�ͬ�����½��У���Ӧ��ϵ��ѹǿ��ʱ��ı仯��ͼ��ʾ��
AX5(g)���ݻ�Ϊ10 L���ܱ������н��С���ʼʱAX3��X2��Ϊ0.2 mol����Ӧ�ڲ�ͬ�����½��У���Ӧ��ϵ��ѹǿ��ʱ��ı仯��ͼ��ʾ��

����ʽ����ʵ��a�ӷ�Ӧ��ʼ���ﵽƽ��ʱ�ķ�Ӧ����v(AX5)��______________________��
��ͼ��3��ʵ��ӷ�Ӧ��ʼ���ﵽƽ��ʱ�ķ�Ӧ����v(AX5)�ɴ�С�Ĵ���Ϊ____________(��ʵ�����)����ʵ��a��ȣ���������ı��ʵ���������ж������ǣ�b________________________________________________��c____________________________________________��
����p0��ʾ��ʼʱ��ѹǿ��p��ʾƽ��ʱ��ѹǿ������ʾAX3��ƽ��ת���ʣ������ı���ʽΪ______________��ʵ��a��c��ƽ��ת���ʣ���aΪ________����cΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10 L�����ܱ������г���X(g)��Y(g)��������ӦX(g)��Y(g)  M(g)��N(g)������ʵ���������±���
M(g)��N(g)������ʵ���������±���
| ʵ�� ��� | �¶�/�� | ��ʼʱ���ʵ���/mol | ƽ��ʱ���ʵ���/mol | ||
| n(X) | n(Y) | n(M) | |||
| �� | 700 | 0.40 | 0.10 | 0.090 | |
| �� | 800 | 0.10 | 0.40 | 0.080 | |
| �� | 800 | 0.20 | 0.30 | a | |
| �� | 900 | 0.10 | 0.15 | b | |
����˵����ȷ����(����)
A��ʵ����У���5 minʱ���n(M)��0.050 mol����0��5 minʱ���ڣ���N��ʾ��ƽ����Ӧ����v(N)��1.0��10��2 mol��L��1��min��1
B��ʵ����У��÷�Ӧ��ƽ�ⳣ��K��2.0
C��ʵ����У��ﵽƽ��ʱ��X��ת����Ϊ60%
D��ʵ����У��ﵽƽ��ʱ��b>0.060
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ijһ����ɱ�ĺ����ܱ������з������·�Ӧ��A(g)��B(g)
 2C(g)����H<0��t1ʱ�̴ﵽƽ�����t2ʱ�̸ı�ijһ�������䷴Ӧ������ͼK221��ʾ������˵����ȷ����(����)
2C(g)����H<0��t1ʱ�̴ﵽƽ�����t2ʱ�̸ı�ijһ�������䷴Ӧ������ͼK221��ʾ������˵����ȷ����(����)
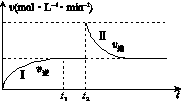
ͼK221
������������������������������������
A��0��t2ʱ��v��>v��
B���������̴ﵽƽ��ʱ��A�����������>��
C��t2ʱ�̸ı���������������ܱ������м�C
D���������̴ﵽƽ��ʱ��ƽ�ⳣ����<��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
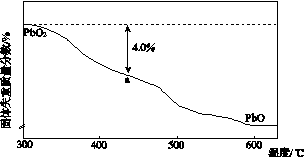
Ǧ���仯������������ء������豸��X���߷������ϵȡ��ش��������⣺
(1)Ǧ��̼��ͬ��Ԫ�أ���̼��4�����Ӳ㡣Ǧ��Ԫ�����ڱ���λ��Ϊ��________���ڡ���________�壻PbO2�����Ա�CO2������________(�ǿ��������)��
(2)PbO2��Ũ���Ṳ�����ɻ���ɫ���壬��Ӧ�Ļ�ѧ����ʽΪ_______________________��
(3)PbO2����PbO�����������Һ��Ӧ�Ƶã���Ӧ�����ӷ���ʽΪ___________________��PbO2Ҳ����ͨ��ʯīΪ�缫��Pb(NO3)2��Cu(NO3)2�Ļ����ҺΪ���Һ�����ȡ�����������ĵ缫��ӦʽΪ____________________�������Ϲ۲쵽��������____________________�������Һ�в�����Cu(NO3)2�����������ĵ缫��ӦʽΪ______________________________������������Ҫȱ����____________________��
(4)PbO2�ڼ��ȹ��̷����ֽ��ʧ����������ͼ��ʾ����֪ʧ�������ϵ�a��Ϊ��Ʒʧ��4.0%(��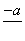 ��100%)�IJ������塣��a�������ɱ�ʾΪPbOx��mPbO2��nPbO����ʽ����xֵ��m��nֵ_______________________________________
��100%)�IJ������塣��a�������ɱ�ʾΪPbOx��mPbO2��nPbO����ʽ����xֵ��m��nֵ_______________________________________
________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com